INTRODUCTION
ORGASMIC FUNCTION: PHYSIOLOGY
PHYSIOPATHOLOGY OF ORGASMIC DYSFUNCTIONS AFTER RADICAL PROSTATECTOMY
PREVALENCE OF ORGASMIC DYSFUNCTIONS AFTER RADICAL PROSTATECTOMY
1. Climacturia
Table 1
Studies reporting the prevalence of climacturia after radical prostatectomy
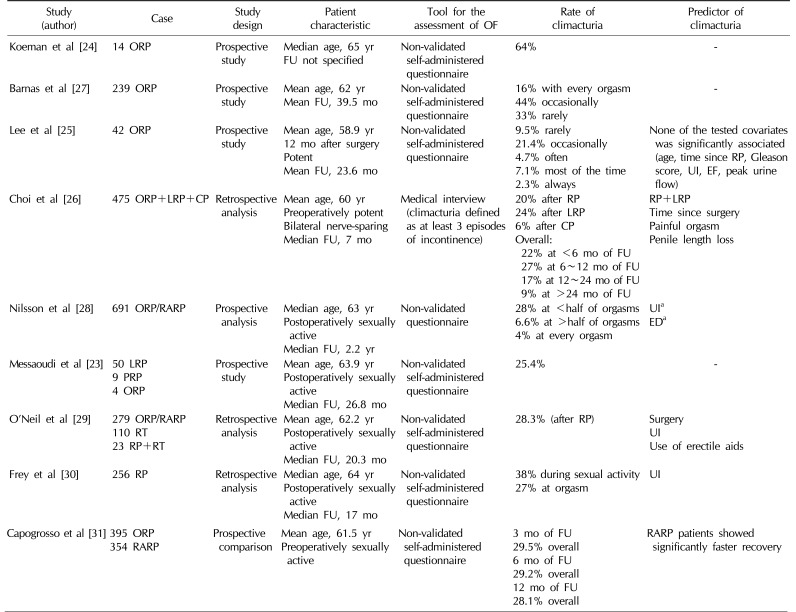
| Study (author) | Case | Study design | Patient characteristic | Tool for the assessment of OF | Rate of climacturia | Predictor of climacturia |
|---|---|---|---|---|---|---|
| Koeman et al [24] | 14 ORP | Prospective study |
Median age, 65 yr FU not specified |
Non-validated self-administered questionnaire | 64% | - |
| Barnas et al [27] | 239 ORP | Prospective study |
Mean age, 62 yr Mean FU, 39.5 mo |
Non-validated self-administered questionnaire |
16% with every orgasm 44% occasionally 33% rarely |
- |
| Lee et al [25] | 42 ORP | Prospective study |
Mean age, 58.9 yr 12 mo after surgery Potent Mean FU, 23.6 mo |
Non-validated self-administered questionnaire |
9.5% rarely 21.4% occasionally 4.7% often 7.1% most of the time 2.3% always |
None of the tested covariates was significantly associated (age, time since RP, Gleason score, UI, EF, peak urine flow) |
| Choi et al [26] | 475 ORP+LRP+CP | Retrospective analysis |
Mean age, 60 yr Preoperatively potent Bilateral nerve-sparing Median FU, 7 mo |
Medical interview (climacturia defined as at least 3 episodes of incontinence) |
20% after RP 24% after LRP 6% after CP Overall: 22% at <6 mo of FU 27% at 6~12 mo of FU 17% at 12~24 mo of FU 9% at >24 mo of FU |
RP+LRP Time since surgery Painful orgasm Penile length loss |
| Nilsson et al [28] | 691 ORP/RARP | Prospective analysis |
Median age, 63 yr Postoperatively sexually active Median FU, 2.2 yr |
Non-validated questionnaire |
28% at <half of orgasms 6.6% at >half of orgasms 4% at every orgasm |
UIa EDa |
| Messaoudi et al [23] |
50 LRP 9 PRP 4 ORP |
Prospective study |
Mean age, 63.9 yr Postoperatively sexually active Median FU, 26.8 mo |
Non-validated self-administered questionnaire | 25.4% | - |
| O’Neil et al [29] |
279 ORP/RARP 110 RT 23 RP+RT |
Retrospective analysis |
Mean age, 62.2 yr Postoperatively sexually active Median FU, 20.3 mo |
Non-validated self-administered questionnaire | 28.3% (after RP) |
Surgery UI Use of erectile aids |
| Frey et al [30] | 256 RP | Retrospective analysis |
Median age, 64 yr Postoperatively sexually active Median FU, 17 mo |
Non-validated self-administered questionnaire | Non-validated self-administered questionnaire | UI |
| Capogrosso et al [31] |
395 ORP 354 RARP |
Prospective comparison |
Mean age, 61.5 yr Preoperatively sexually active |
Non-validated self-administered questionnaire |
3 mo of FU 29.5% overall 6 mo of FU 29.2% overall 12 mo of FU 28.1% overall |
RARP patients showed significantly faster recovery |
OF: orgasmic function, ORP: open radical prostatectomy, LRP: laparoscopic radical prostatectomy, CP: cystoprostatectomy, RARP: robot-assisted radical prostatectomy, PRP: perineal radical prostatectomy, RT: radiotherapy, RP: radical prostatectomy, FU: follow-up, UI: urinary incontinence, ED: erectile dysfunction.
aUnivariate analysis.
2. Painful orgasm
Table 2
Studies reporting the prevalence of painful orgasm after radical prostatectomy
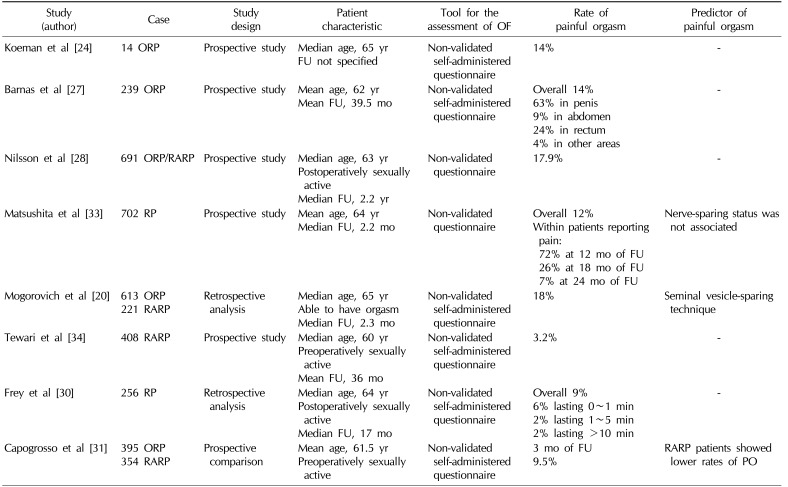
| Study (author) | Case | Study design | Patient characteristic | Tool for the assessment of OF | Rate of painful orgasm | Predictor of painful orgasm |
|---|---|---|---|---|---|---|
| Koeman et al [24] | 14 ORP | Prospective study |
Median age, 65 yr FU not specified |
Non-validated self-administered questionnaire | 14% | - |
| Barnas et al [27] | 239 ORP | Prospective study |
Mean age, 62 yr Mean FU, 39.5 mo |
Non-validated self-administered questionnaire |
Overall 14% 63% in penis 9% in abdomen 24% in rectum 4% in other areas |
- |
| Nilsson et al [28] | 691 ORP/RARP | Prospective study |
Median age, 63 yr Postoperatively sexually active Median FU, 2.2 yr |
Non-validated questionnaire | 17.9% | - |
| Matsushita et al [33] | 702 RP | Prospective study |
Mean age, 64 yr Median FU, 2.2 mo |
Non-validated questionnaire |
Overall 12% Within patients reporting pain: 72% at 12 mo of FU 26% at 18 mo of FU 7% at 24 mo of FU |
Nerve-sparing status was not associated |
| Mogorovich et al [20] |
613 ORP 221 RARP |
Retrospective analysis |
Median age, 65 yr Able to have orgasm Median FU, 2.3 mo |
Non-validated self-administered questionnaire | 18% | Seminal vesicle-sparing technique |
| Tewari et al [34] | 408 RARP | Prospective study |
Median age, 60 yr Preoperatively sexually active Mean FU, 36 mo |
Non-validated self-administered questionnaire | 3.20% | - |
| Frey et al [30] | 256 RP | Retrospective analysis |
Median age, 64 yr Postoperatively sexually active Median FU, 17 mo |
Non-validated self-administered questionnaire |
Overall 9% 6% lasting 0~1 min 2% lasting 1~5 min 2% lasting >10 min |
- |
| Capogrosso et al [31] |
395 ORP 354 RARP |
Prospective comparison |
Mean age, 61.5 yr Preoperatively sexually active |
Non-validated self-administered questionnaire |
3 mo of FU 9.5% |
RARP patients showed lower rates of PO |
3. Alterations of orgasmic sensation
Table 3
Studies reporting the prevalence of altered orgasmic sensation after radical prostatectomy
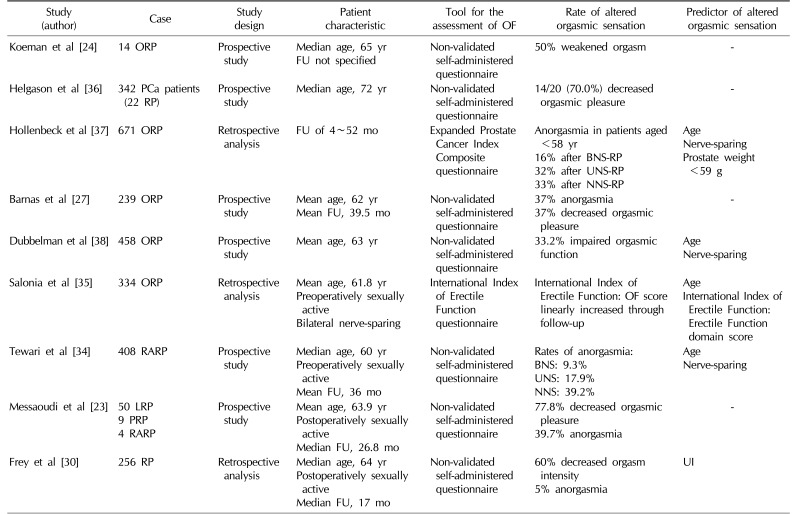
| Study (author) | Case | Study design | Patient characteristic | Tool for the assessment of OF | Rate of altered orgasmic sensation | Predictor of altered orgasmic sensation |
|---|---|---|---|---|---|---|
| Koeman et al [24] | 14 ORP | Prospective study |
Median age, 65 yr FU not specified |
Non-validated self-administered questionnaire | 50% weakened orgasm | - |
| Helgason et al [36] | 342 PCa patients (22 RP) | Prospective study | Median age, 72 yr | Non-validated self-administered questionnaire | 14/20 (70.0%) decreased orgasmic pleasure | - |
| Hollenbeck et al [37] | 671 ORP | Retrospective analysis | FU of 4~52 mo | Expanded Prostate Cancer Index Composite questionnaire |
Anorgasmia in patients aged <58 yr 16% after BNS-RP 32% after UNS-RP 33% after NNS-RP |
Age Nerve-sparing Prostate weight <59 g |
| Barnas et al [27] | 239 ORP | Prospective study |
Mean age, 62 yr Mean FU, 39.5 mo |
Non-validated self-administered questionnaire |
37% anorgasmia 37% decreased orgasmic pleasure |
- |
| Dubbelman et al [38] | 458 ORP | Prospective study | Mean age, 63 yr | Non-validated self-administered questionnaire | 33.2% impaired orgasmic function |
Age Nerve-sparing |
| Salonia et al [35] | 334 ORP | Retrospective analysis |
Mean age, 61.8 yr Preoperatively sexually active Bilateral nerve-sparing |
International Index of Erectile Function questionnaire | International Index of Erectile Function: OF score linearly increased through follow-up |
Age International Index of Erectile Function: Erectile Function domain score |
| Tewari et al [34] | 408 RARP | Prospective study |
Median age, 60 yr Preoperatively sexually active Mean FU, 36 mo |
Non-validated self-administered questionnaire |
Rates of anorgasmia: BNS: 9.3% UNS: 17.9% NNS: 39.2% |
Age Nerve-sparing |
| Messaoudi et al [23] |
50 LRP 9 PRP 4 RARP |
Prospective study |
Mean age, 63.9 yr Postoperatively sexually active Median FU, 26.8 mo |
Non-validated self-administered questionnaire |
77.8% decreased orgasmic pleasure 39.7% anorgasmia |
- |
| Frey et al [30] | 256 RP | Retrospective analysis |
Median age, 64 yr Postoperatively sexually active Median FU, 17 mo |
Non-validated self-administered questionnaire |
60% decreased orgasm intensity 5% anorgasmia |
UI |
OF: orgasmic function, ORP: open radical prostatectomy, PCa: prostate cancer, RP: radical prostatectomy, RARP: robot-assisted radical prostatectomy, LRP: laparoscopic radical prostatectomy, PRP: perineal radical prostatectomy, FU: follow-up, BNS: bilateral nerve-sparing, UNS: unilateral nerve-sparing, NNS: non-nerve-sparing, UI: urinary incontinence.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download