Abstract
Purpose
This study was designed to evaluate the efficacy of medical treatment of Peyronie's disease.
Materials and Methods
A total of 109 patients with Peyronie's disease who had been treated from January 2011 to December 2014 were retrospectively reviewed in this study. Forty-four patients (Group 1) were treated with 12 mg of potassium para-aminobenzoate daily. Sixty-five patients (Group 2) were treated with combination therapy: tamoxifen (20 mg) and acetyl-L-carnitine (300 mg) twice daily in addition to a phosphodiesterase type 5 inhibitor. Ability to perform sexual intercourse, pain during erection, size of plaque, and penile curvature angle were assessed.
Results
In Group 1, 30 of 44 patients (68.2%) discontinued treatment within 12 weeks, while 5 patients (7.7%) in Group 2 discontinued treatment. Pain during erection and plaque size were improved in both groups but showed no statistical difference due to the high dropout rate in Group 1. In both groups, penile curvature was improved, but demonstrated no statistical difference between the treatment groups. However, combination therapy demonstrated a better response rate in patients whose penile curvature angle was less than 30° (44.4% vs. 79.1%, p=0.048). The rate of successful sexual intercourse was significantly higher in Group 2 (42.8% vs. 78.3%, p=0.034). The number of patients who underwent surgical correction despite medical treatment was significantly higher in Group 1 (35.7% vs. 13.3%, p=0.048).
Peyronie's disease is a localized connective tissue disorder that involves tunica albuginea of the penis [1]. Peyronie's disease may present signs and symptoms including penile curvature, pain on erection, erectile dysfunction, and plaque formation. Studies of the prevalence of Peyronie's disease have determined ranges from 0.7% to 9.0% of men [234]. Peyronie's disease mainly affects those in their sixties and seventies. The actual prevalence of Peyronie's disease is considered to be higher because of patients' reluctance to visit a clinic to diagnose and treat this condition.
There are conservative, non-surgical options and surgical options for the treatment of Peyronie's disease. The surgical option remains the most effective treatment [56]. However, surgical correction cannot be recommended for all patients at time of first visit, particularly for those who are elderly.
To be a surgical candidate, the patient's disease should be stabilized, with no pain and no aggravation of penile curvature for at least 3 months. The patient's desire for active intercourse is also important, as the primary aim of surgery is to allow satisfactory intercourse by correcting the curvature [6]. Therefore, we believe that the primary goal of conservative management is to preserve or improve the ability to have successful sexual intercourse by improving the curvature or preventing it from worsening. In practice, most patients are asked to follow a course of medical treatment for at least 6 months.
Various oral medical agents have been tested for treatment of Peyronie's disease, including vitamin E (tocopherol), oral colchicine, potassium para-aminobenzoate, tamoxifen, L-carnitine, and phosphodiesterase type 5 (PDE5) inhibitors.
Potassium para-aminobenzoate (Potaba; Glenwood, LLC, Englewood, NJ, USA) was approved as a treatment option for Peyronie's disease in 1959 by the United States Food and Drug Administration, and became commercially available in South Korea from 2011 [7]. Tamoxifen is reported to be effective for decreasing pain and plaque size in Peyronie's disease, although some studies reported no efficacy over placebo group [89]. L-carnitine, the natural precursor of acetylcholine, is reported to decrease pain and inhibit the progression of Peyronie's disease. It is relatively safe, with rare adverse events [10]. PDE5 inhibitors are widely used drugs for the treatment of erectile dysfunction. PDE5 inhibitors also have shown preventive and corrective effects in rat models of Peyronie's disease. Some studies reported that PDE5 inhibitors might have beneficial effects on Peyronie's disease in men [1112]. However, no traditional single- or dual-agent therapy has proven to have definite efficacy for Peyronie's disease to date.
We treated patients with Peyronie's disease with potassium para-aminobenzoate therapy or combination therapy consisting of tamoxifen, L-carnitine, and a PDE5 inhibitor. We retrospectively reviewed and analyzed the efficacy of these treatments in Peyronie's disease.
After the Institutional Review Board of Korea University Guro Hospital approved the study protocol, we retrospectively reviewed the medical records of Peyronie's disease in our clinic from January 2011 to December 2014. A total of 109 patients with Peyronie's disease were treated with either potassium para-aminobenzoate or combination therapy. Forty-four patients (Group 1) received 3 g of potassium para-aminobenzoate (500 mg capsule) four times daily. Sixty-five patients (Group 2) received treatment with 20 mg of tamoxifen and 330 mg acetyl-L-carnitine twice daily in addition to a PDE5 inhibitor (tadalafil, 5 mg once daily). Every patient was instructed to take vitamin E daily.
We evaluated the history of underlying disease in the patients, the subjective pain during erection using a visual analogue scale (0: no pain, 5: moderate pain, 10: worst pain), the plaque size, and the degree of penile curvature. The maximal longitudinal length of plaque size was measured (in mm) with penile ultrasonography. The degree of the penile curvature angle was measured with a protractor. These measurements were performed during erection induced by prostaglandin E1 (10 to 20 µg). All patients were questioned as to ability to perform sexual intercourse, vaginal penetration, and erection maintenance.
Treatment duration was at least 3 months and the follow-up duration was at least 12 weeks after active therapy. After treatment for 6 months, we assessed and analyzed the treatment outcomes.
Primary treatment goal was assessed by feasibility of sexual intercourse despite residual curvature. Occurrence of side effects and dropout rate at the time were also investigated. Thirty percent or more reduction in the degree of angle was defined as a response in penile curvature.
We compared those responses between the treatment groups with the Student's t-test for a continuous, parametric variable; we used a chi-square and Mann-Whitney U-test for other variables. IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. A p-value less than 0.05 was considered significant.
The mean age of each group was 57.8±9.4 years in Group 1, and 59.3±8.7 years in Group 2, respectively (p=0.439). In 20 of 44 patients (45.5%) in Group 1 and 22 of 65 patients (33.8%) in Group 2, ability to penetrate the vagina and maintain erection during intercourse was impaired or impossible, respectively (p=0.164).
In Group 1, 16 of 44 patients (36.4%) presented pain during erection while 32 of 65 patients (49.2%) in Group 2 presented pain (p=0.184).
The longitudinal maximal plaque size was 16.8±7.2 mm and 17.0±5.0 mm, respectively (p=0.122). The degree of penile curvature angle of Group 1 was 23.7°±16.6°, and that of Group 2 was 27.4°±18.6°, respectively (p=0.321).
The differences in baseline characteristics between the treatment groups were not significant (Table 1).
During the treatment, a total of 35 patients (32.1%) aborted the therapy within 12 weeks. Thirty of 44 patients (68.2%) in Group 1 (potassium para-aminobenzoate) discontinued therapy: 11 patients (36.7%) for gastrointestinal distress; 10 patients (33.3%) due to too many drugs; 6 patients (20.0%) due to little or no effectiveness; and 3 patients (10.0%) due to relatively high cost (Table 2).
In Group 2, 5 patients (7.7%) discontinued therapy within 12 weeks: 2 patients (40.0%) due to gastrointestinal distress; 2 patients (40.0%) due to high cost; and one patient (20.0%) due to too many drugs. Dropout and loss of follow-up rate for Group 1 was significantly higher than Group 2 (p<0.001).
A total of 13 patients, 5 patients (5/14, 35.7%) in Group 1, and 8 patients (8/60, 13.3%) in Group 2, received surgical correction for dissatisfaction despite medical treatment (p=0.048).
After the exclusion of the 35 patients who dropped out, duration of treatment was not different between the two treatment groups (142.3±49.6 days vs. 159.8±71.7 days, p=0.148). Comparing ability for sexual intercourse, the number of patients who were able to perform intercourse successfully after treatment was significantly higher in Group 2 (6 patients vs. 47 patients, 42.8% vs. 78.3%, p=0.034). The rate of pain during erection in Group 2 (combination therapy) decreased to 15.0%. But this outcome was not significantly different in comparison to Group 1 (p=0.687) (Table 3).
The response rate in regard to penile curvature angle did not change by treatment (p=0.154). Combination therapy appeared to show a better result than potassium para-aminobenzoate therapy in terms of improved curvature in the subgroup whose penile angle was less than 30° (44.4% vs. 79.1%, p=0.048). There was no significant difference in regard to decreased plaque size (p=0.468).
In this study, we evaluated the efficacy of potassium para-aminobenzoate monotherapy vs. combination therapy consisting of tamoxifen, L-carnitine, and a PDE5 inhibitor for the preoperative treatment of Peyronie's disease in terms of tolerable sexual intercourse as primary endpoint and secondary endpoints such as improvement of pain, penile angle, and plaque size.
Potassium para-aminobenzoate (Potaba) is believed to have an antifibrotic effect by decreasing the concentration of serotonin, which contributes to fibrogenesis [7]. Since Zarafonetis and Horrax [7] first reported that potassium para-aminobenzoate demonstrated improvement of pain, penile angle, and plaque size, many studies have been conducted to confirm these data. Shah et al [13] reported in a prospective double-blind study that potassium paraaminobenzoate significantly reduced penile pain. Another double-blind, placebo-controlled study reported that potassium para-aminobenzoate decreased plaque size significantly but was not effective for penile curvature and penile pain [14]. Although potassium para-aminobenzoate could not improve preexisting penile curvature, it appears to be protective against deterioration of penile curvature.
The European Association of Urology guideline for penile curvature gives a grade B recommendation ('based on well-conducted clinical studies, but without randomised clinical trial') of potassium para-aminobenzoate to reduce penile plaque size and pain in Peyronie's disease [6]. Potaba was introduced and permitted for use in Korea in 2011 for treatment of Peyronie's disease, and has gained popularity in recent years.
Unlike another study in which potassium para-aminobenzoate was reported to cause fewer side effects [14], we observed many adverse events and poor compliance. While the study demonstrated a 7.8% dropout rate for adverse events, our study showed that two-thirds of patients aborted therapy, 36% of which was due to gastrointestinal distress after taking drugs. Excessive frequency and number of doses also contributed to poor compliance. We used potassium para-aminobenzoate only temporarily, due to poor compliance. Although we cannot be certain why the rate of adverse events in our study is so high, considering that other studies about the efficacy of potassium para-aminobenzoate have been based on non-Asian populations, the ethnicity of the patients may be a related factor. We speculate that the efficient therapeutic dosage of potassium para-aminobenzoate may be different in Asian people or some ethnicities in Asia, such as the Koreans studied here. We suggest that further research may be needed to determine a way of lowering the adverse event rate and the sufficient therapeutic dosage for people of Asian origin.
Tamoxifen inhibits the release of transforming growth factor alpha and beta from human fibroblast, which leads to reduced inflammatory response in lesions and reduced plaque size through the inhibition of angiogenesis and fibrogenesis [15]. Ralph et al [8] first reported that tamoxifen is effective for Peyronie's disease in 1992. In their study, 80% of patients showed the resolution of pain and 34% of patients reported reduced plaque size. However, a recent placebo-controlled study reported that tamoxifen demonstrated no therapeutic advantage over a placebo-control group [9].
Acetyl-L-carnitine is the natural precursor of acetylcholine, which is normally synthesized in the human brain, liver, and kidney [16]. L-carnitine is reported to be relatively safe for its few side effects, except some occasional mild euphoria [10]. L-carnitine is hypothesized to prevent proliferation of fibroblast and collagenogenesis by reducing free radicals and intracellular calcium concentration. It also protects and restores cells with damage caused by inflammation and ischemia [17]. In a randomized comparison study of L-carnitine to tamoxifen, L-carnitine showed better improvement in penile pain and curvature angle than tamoxifen [16]. Cavallini et al [18] reported a study that oral L-carnitine plus verapamil injection reduced penile curvature and plaque size in comparison with oral tamoxifen plus verapamil injection. However, a recent study reported that neither L-carnitine monotherapy nor L-carnitine with vitamin E combination therapy showed a beneficial effect over a placebo group [19].
PDE5 inhibitors are speculated to prevent plaque development and reduce its size through maintaining a high level of cyclic guanosine monophosphate in the target tissue, which has an anti-fibrotic effect [20]. Valente et al [11] reported that continuous administration of a PDE5 inhibitor to rats prevented the formation of fibrotic plaque and decreased plaque size. In the study, the PDE5 inhibitor stimulated the apoptosis of fibroblasts. In another study, the addition of tadalafil to extracorporeal shockwave lithotripsy (ESWL) resulted in a significantly better International Index of Erectile Function score and quality of life score than with ESWL therapy alone [21].
It has been reported that 30% to 70% of Peyronie's patients may have erectile dysfunction [2223]. The decrease of rigidity and curvature, negative self-impression, and anxiety caused by Peyronie's disease may contribute to erectile dysfunction. In addition, Peyronie's disease may cause the development of negative hemodynamic instability. One study reported that penile vascular abnormality was observed in 76.5% of cases with Peyronie's disease [24].
Levine and colleagues reported that 98.9% of patients with Peyronie's disease complained of a decrease in erectile capacity occurring before and after the onset of the disease, and 58.5% patients sought treatment for erectile dysfunction [25]. In the study, the author treated patients with sildenafil citrate and 70.8% of patients were satisfied with the treatment. The author was concerned about the deterioration of the disease due to minor trauma during intercourse. However, no patients showed worsening of penile deformity or an increase in penile pain. Despite the potential risk of coital trauma to the penis, considering this study, a PDE5 inhibitor appears to be safe. It seems that more active use of a PDE5 inhibitor in Peyronie's disease may be justifiable.
In this study, we aimed to analyze and compare our experience of oral drug therapy in the treatment of Peyronie's disease. We hypothesized that combination drug therapy, covering each different mechanism of drug action, may be more effective to ameliorate or prevent deterioration of the disease. Although not all treatment outcomes have beneficial effects, combination therapy showed better results with regard to some factors. The results of our study suggest that combination therapy has a more beneficial effect in regard to successful sexual intercourse over potassium para-aminobenzoate therapy. Regarding the penile curvature response rate, combination therapy did not have beneficial effects over potassium para-aminobenzoate therapy in all patients. However, combination therapy appears to produce a better response in those patients whose curvature angle is mild, particularly when it is less than 30°. This could be noteworthy, for it suggests that early treatment may lead to better results. Combination therapy also improved the ability to perform sexual intercourse successfully, thus making surgical correction less of a necessity. In fact, we observed a significantly lower rate of surgical correction after medical therapy in the combination therapy group. We conjecture that this may have been due to increased sexual self-confidence and actual hemodynamic improvement in the penis, caused by the PDE5 inhibitor.
Our study has some limitations. Because it is a retrospective and non-randomized study, treatment selection of this study may be biased by the preference of the patient or the severity of the symptoms. The power of analysis may also be limited for lack of sufficient sample size. Furthermore, our study lacks a placebo group, thus making it difficult to recognize the real impact of drug therapy. Considering the natural course of Peyronie's disease, however, which usually shows no change or worsens without treatment, it was difficult to arrange a placebo-control group in clinical practice for ethical reasons.
In the future, a double-blind, randomized, prospective study with a greater sample size may be necessary. The reason for poor compliance with potassium para-aminobenzoate treatment, and ways of altering the dose and usage to cope with it, also need to be determined.
Figures and Tables
Table 1
The baseline characteristics of patients before treatment

Table 2
Dropout rate, cause of failure, and rate of convertsion to surgery
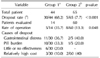
Table 3
Comparison of each treatment outcomes

Values are presented as number only, mean±standard deviation, number (%), or number/total number (%). p-value <0.05 was considered to bestatistically significant.
aPotassium para-aminobenzoate monotherapy. bCombination therapy with tamoxifen, L-carnitine, and phosphodiesterase type 5 inhibitor. cΔPlaque size means net change of plaque size after treatment.
References
1. Hellstrom WJ, Bivalacqua TJ. Peyronie's disease: etiology, medical, and surgical therapy. J Androl. 2000; 21:347–354.
2. Smith CJ, McMahon C, Shabsigh R. Peyronie's disease: the epidemiology, aetiology and clinical evaluation of deformity. BJU Int. 2005; 95:729–732.

3. Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie's disease: results of a large survey. BJU Int. 2001; 88:727–730.

4. Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010; 7:215–221.

5. Segal RL, Burnett AL. Surgical management for Peyronie's disease. World J Mens Health. 2013; 31:1–11.

6. Hatzimouratidis K, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Salonia A, et al. European Association of Urology. EAU guidelines on penile curvature. Eur Urol. 2012; 62:543–552.

7. Zarafonetis CJ, Horrax TM. Treatment of Peyronie's disease with potassium para-aminobenzoate (potaba). J Urol. 1959; 81:770–772.

8. Ralph DJ, Brooks MD, Bottazzo GF, Pryor JP. The treatment of Peyronie's disease with tamoxifen. Br J Urol. 1992; 70:648–651.

9. Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol. 1999; 162:2003–2005.

10. Bonavita E. Study of the efficacy and tolerability of L-acetylcarnitine therapy in the senile brain. Int J Clin Pharmacol Ther Toxicol. 1986; 24:511–516.
11. Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003; 9:229–244.

12. Chung E, Deyoung L, Brock GB. The role of PDE5 inhibitors in penile septal scar remodeling: assessment of clinical and radiological outcomes. J Sex Med. 2011; 8:1472–1477.

13. Shah PJR, Green NA, Adib RS, Stewart PAH, Smith P, Coxon JG, et al. A multicentre double blind controlled trial of potassium paraaminobenzoate (Potaba) in Peyronie's disease. Prog Reprod Biol Med. 1983; 9:61–67.
14. Weidner W, Hauck EW, Schnitker J. Peyronie's Disease Study Group of Andrological Group of German Urologists. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol. 2005; 47:530–535.

15. Wahl SM, McCartney-Francis N, Mergenhagen SE. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989; 10:258–261.
16. Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: a preliminary report. BJU Int. 2001; 88:63–67.

17. Jack GS, Gonzalez-Cadavid N, Rajfer J. Conservative management options for Peyronie's disease. Curr Urol Rep. 2005; 6:454–460.

18. Cavallini G, Biagiotti G, Koverech A, Vitali G. Oral propionyl-l-carnitine and intraplaque verapamil in the therapy of advanced and resistant Peyronie's disease. BJU Int. 2002; 89:895–900.

19. Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: a double-blind, placebo controlled, randomized study. J Urol. 2007; 178:1398–1403.

20. Ferrini MG, Kovanecz I, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int. 2006; 97:625–633.

21. Palmieri A, Imbimbo C, Creta M, Verze P, Fusco F, Mirone V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie's disease and erectile dysfunction: results from a prospective randomized trial. Int J Androl. 2012; 35:190–195.

22. Wunderlich H, Werner W, Schubert J. Coincidence of induratio penis plastica and erectile dysfunction. Urol Int. 1998; 60:97–100.

23. Weidner W, Schroeder-Printzen I, Weiske WH, Vosshenrich R. Sexual dysfunction in Peyronie's disease: an analysis of 222 patients without previous local plaque therapy. J Urol. 1997; 157:325–328.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download