Abstract
Purpose
The goal of this study was to investigate the association between hypercholesterolemia and the time required for progression to castration-resistant prostate cancer (CRPC) in patients who have undergone androgen deprivation therapy (ADT).
Materials and Methods
Data from 154 patients with prostate cancer between 2005 and 2012 were reviewed retrospectively. ADT was employed as a treatment modality for these patients either due to multiple bone metastases at the time of diagnosis or due to old age in combination with other morbidities. Serum cholesterol levels and statin use were reviewed. We analyzed the factors associated with the development of CRPC after ADT treatment. The mean follow-up period was 34.8 months.
Results
The mean age of the patients was 71.3 years old and their mean prostate-specific antigen level was 141.8±212.6 ng/mL. Their mean cholesterol level was 175.9±37.7 mg/dL, and 14 patients (9.1%) were statin users. CRPC developed in 44 patients (28.6%), and the mean duration from ADT treatment to CRPC was 24.1 months. In a multivariate analysis, hypercholesterolemia was associated with the development of CRPC (hazard ratio [HR]=1.017, p<0.001), depending on clinical T stage (p=0.005) and the presence of bone metastasis (p<0.001). A subanalysis showed that hypercholesterolemia was associated with the development of CRPC in patients with bone metastasis (HR=1.032, p<0.001), but not in patients without bone metastasis.
Prostate cancer is the second leading cause of cancer death in men in the United States [1]. Prostatectomy and radiation therapy are the preferred treatment modalities in men with localized prostate cancer. Androgen deprivation therapy (ADT) is used as an adjuvant therapy after radical prostatectomy and for the treatment of metastatic prostate cancer. Although prostate cancer is generally sensitive to initial ADT, tumors that have overcome the absence of gonadal androgens eventually regrow [2]. When prostate cancer progresses despite castration levels of testosterone, it is termed castration-resistant prostate cancer (CRPC) a term that reflects the knowledge that such cases of re-emergent prostate cancer are not independent of androgens, as was once believed. Unfortunately, recurrent tumors are more aggressive, evasive, and deadly [3], and most prostate cancer deaths are from CRPC.
Cholesterol has been implicated in the pathogenesis of diseases such as cardiovascular disease and in many forms of cancer, including prostate cancer [4]. Prostate cancer is associated with elevated levels of cholesterol, which may support cell proliferation by contributing cholesterol for membrane composition and signal transduction [5,6]. Based on these findings, several studies have explored the relationship between serum cholesterol levels and the incidence of prostate cancer and associated mortality. However, these studies have reported inconsistent results [7,8,9,10,11]. Similarly, the effects of statins on the overall risk reduction of prostate cancer remains controversial [12,13,14], with some studies suggesting that statins have no effect, while others have found statins to be associated with significant risk reduction, especially in advanced-stage tumors [15,16]. However, previous research has not addressed the relationship between levels of cholesterol and tumor progression to CRPC in patients with prostate cancer.
In this study, we aimed to investigate the potential association between hypercholesterolemia and the risk of progression to CRPC in men who have undergone ADT.
After Institutional Review Board approval in Ulsan University Hospital (No. 2015-06-009), data from 314 patients who were diagnosed with prostate cancer from 2005 to 2012 were reviewed retrospectively. After excluding 160 patients who underwent radical prostatectomy or were transferred to other clinics, 154 patients who were initially treated with ADT were included. ADT was employed as a treatment modality for these patients either due to multiple bone metastases at the time of diagnosis (n=50, 32.5%) or due to old age accompanied by other morbidities (n=104, 67.5%), instead of radical prostatectomy or radiotherapy. ADT involved bilateral orchiectomy in nine patients and maximal androgen blockade including luteinizing hormone-releasing hormone agonist injection and oral antiandrogens in 145 patients. The primary outcome was the time to prostate-specific antigen (PSA) progression. In patients with castration serum levels of testosterone (<50 ng/dL), the time to PSA progression indicative of CRPC while undergoing ADT was defined as the time from the initiation of ADT to a 50% increase in PSA from its lowest level or the time from the initiation of ADT to a 25% increase in PSA levels from baseline, and was confirmed using PSA consensus criteria [17]. The mean follow-up was 34.8 months (median, 25.4 months).
Patients' levels of serum cholesterol at diagnosis and statin use were reviewed. The levels of serum cholesterol were available in all patients. Cholesterol levels were categorized as hypercholesterolemia (>240 mg/dL, n=8), borderline (200~240 mg/dL, n=40) and desirable (<200 mg/dL, n=106). High- and low-density lipoprotein (HDL and LDL) cholesterol levels were not present for all patients and, therefore, were not included in the analysis. Patients who took statins before being diagnosed with prostate cancer were categorized as statin users. The statins used in these patients included atorvastatin (n=6), simvastatin (n=4), pravastatin (n=2), and rosuvastatin (n=2). The doses of statin were translated into dose equivalents based on previous published study [18], with 20 mg of simvastatin assigned a value of 1. Dose equivalents of <1, 1, and >1 were present in two patients (14.3%), nine patients (64.3%), and three patients (21.4%), respectively. The duration of statin use was not evaluated.
Clinicopathological characteristics were compared between patients with and without bone metastasis at the time of diagnosis using the chi-square test and the Student's t-test. Multivariate analysis was performed to evaluate the factors associated with the development of CRPC after ADT treatment. Since statin use and cholesterol could affect each other, multivariate analysis was performed again, including statin use instead of cholesterol. A subanalysis was performed according to the presence of bone metastasis at the initial diagnosis. PSA levels were measured every three months after ADT treatment. Radiographic evaluation using abdominopelvic computed tomography or pelvic magnetic resonance and radionuclide bone scanning was performed every six months and when clinically indicated. None of the patients experienced severe complications requiring the discontinuation of ADT therapy. SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, with p-values <0.05 considered to indicate statistical significance.
The mean age of the patients was 71.3 years old, and their mean PSA level was 141.8±212.6 ng/mL at the time of diagnosis (range, 1.5~876 ng/mL). The clinical T stage was T1 or T2 in 72 patients (46.8%), T3 in 64 patients (41.6%), and T4 in 18 patients (11.7%). The Gleason score (GS) was ≤6 in 34 patients (22.1%), 7 in 62 patients (40.3%), and ≥8 in 58 patients (37.7%). The mean level of serum cholesterol was 175.9±37.7 mg/dL and 14 patients (9.1%) were statin users. Patients without bone metastasis had lower PSA levels (84.8 ng/mL vs. 304.6 ng/mL) and lower cholesterol levels (172.4 mg/dL vs. 185.9 mg/dL) than those with bone metastasis (Table 1). Furthermore, patients without bone metastasis had lower GSs and less advanced T stage findings than patients with bone metastasis.
CRPC developed in 44 patients (28.6%) and the mean duration from ADT treatment to CRPC was 24.1 months. CRPC developed in 16 patients (10.4%) of the patients without bone metastasis, with a mean duration of 24.8 months, and 10 patients exhibited newly developed bone metastasis when CRPC was diagnosed. In contrast, CRPC developed in 28 patients (56.0%) with bone metastasis, with a mean duration of 23.6 months, and all patients in this group exhibited aggravated metastatic bone lesions when CRPC was diagnosed. In multivariate analysis, hypercholesterolemia was associated with the development of CRPC (hazard ratio [HR]=1.017, p<0.001), depending on the clinical T stage (HR=4.493, p=0.005 for T3 vs. HR=15.98, p<0.001 for T4), and the presence of bone metastasis (HR=7.578, p<0.001) (Table 2). Statin use was not associated with the development of CRPC. In the subanalysis, hypercholesterolemia was associated with the development of CRPC in patients with bone metastasis at diagnosis (HR=1.032, p<0.001) (Table 3). However, in patients without bone metastasis, hypercholesterolemia was not associated with the development of CRPC (Table 4).
In this cohort, patients without bone metastasis were found to have lower cholesterol levels than patients with bone metastasis. Our findings are comparable with those from other studies, which have shown an inverse association between statin use and the risk of advanced-stage and high-grade disease, with or without M1 lesions [15,16,19,20]. After excluding statin users, the inverse associations for high-grade tumors persisted. This finding suggests that cholesterol itself may play a role in pathogenesis. The mechanisms through which statins inhibit cancer processes can be classified into cholesterol-mediated and noncholesterol-mediated mechanisms [12]. Statins decrease cholesterol levels by inhibiting HMG-CoA reductase. Cholesterol is a major component of lipid rafts, which are involved in signaling pathways regulating prostate cancer cell survival and proliferation [12,21]. Additionally, statins may influence the rate of prostate cancer progression by inhibiting the synthesis of isoprenoids. In observational studies, statin use has been found to be associated with a reduced risk of aggressive prostate cancer [15,16]. However, previous studies analyzing the relationship between cholesterol levels and the incidence of aggressive prostate cancer have reported inconsistent conclusions [7,8,9,10,11]. A possible explanation for these discrepancies is the possibility that cholesterol levels in the environment of the tumor may not be reflective of serum levels.
The present study found hypercholesterolemia to be associated with the development of CRPC after ADT in patients with bone metastasis at the time of the initial diagnosis of prostate cancer. Similarly, a previous study evaluating the association of cholesterol levels with grades of prostate cancer found that men with higher cholesterol levels (>240 mg/dL) were more likely to develop highgrade or rapidly growing metastatic prostate cancer than men with desirable (<200 mg/dL) or borderline levels (200~240 mg/dL) [9]. However, in the present study, it was found that the development of CRPC was not associated with the presence of high, borderline, or desirable cholesterol levels. While cholesterol levels have been reported to increase concomitantly with the transition from non-cancerous to cancerous prostate epithelial cells [22], little is known about how this correlates with the progression to CRPC. A previous study reported that cholesterol homeostasis can be maintained during the progression of prostate cancer [23]. If CRPC cells maintain cholesterol homeostasis, cholesterol accumulation will occur during the progression to the advanced stage of prostate cancer. Thus, hypercholesterolemia at the time of prostate cancer diagnosis might imply that the disease has progressed. However, the relationship between hypercholesterolemia and tumor progression has not yet been fully elucidated. It has not been conclusively determined whether initiating cholesterol-lowering drugs (statins) after the diagnosis of prostate cancer is capable of delaying disease progression.
In this study, statin use was not associated with the development of CRPC (data not shown). Furthermore, the type and dose of statin were not associated with the development of CRPC. However, considering the cholesterol-lowering effect of statins and the fact that previous studies have demonstrated an association between statins and less progressive prostate cancer, it is possible that further studies with larger populations may lead to meaningful results.
This study has some limitations. First, the present study included a relatively small sample size and was a retrospective study. Second, LDL and HDL cholesterol levels were not analyzed. LDL cholesterol levels are more strongly associated with prostate cancer recurrence after radiotherapy than total cholesterol levels [24]. Nonetheless, our study suggested that hypercholesterolemia may be associated with the development of CRPC in patients with bone metastasis at the time of diagnosis. Clinicians may wish to consider cholesterol levels as a prognostic factor in metastatic prostate cancer before starting ADT treatment.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249. PMID: 19474385.

2. Goldenberg SL, Gleave ME, Taylor D, Bruchovsky N. Clinical experience with intermittent androgen suppression in prostate cancer: minimum of 3 years' follow-up. Mol Urol. 1999; 3:287–292. PMID: 10851335.
3. Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001; 1:34–45. PMID: 11900250.

4. Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006; 9:379–385. PMID: 16778565.

5. Di Vizio D, Solomon KR, Freeman MR. Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori. 2008; 94:633–639. PMID: 19112935.

6. Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004; 91:54–69. PMID: 14689582.

7. Batty GD, Kivimäki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011; 22:311–318. PMID: 21116843.

8. Iso H, Ikeda A, Inoue M, Sato S, Tsugane S. JPHC Study Group. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009; 125:2679–2686. PMID: 19544528.

9. Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010; 21:61–68. PMID: 19806465.

10. Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008; 123:1693–1698. PMID: 18646186.

11. Bravi F, Scotti L, Bosetti C, Talamini R, Negri E, Montella M, et al. Self-reported history of hypercholesterolaemia and gallstones and the risk of prostate cancer. Ann Oncol. 2006; 17:1014–1017. PMID: 16611646.

12. Papadopoulos G, Delakas D, Nakopoulou L, Kassimatis T. Statins and prostate cancer: molecular and clinical aspects. Eur J Cancer. 2011; 47:819–830. PMID: 21354784.

13. Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008; 17:27–36. PMID: 17944002.

14. Murtola TJ, Tammela TL, Määttänen L, Huhtala H, Platz EA, Ala-Opas M, et al. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int J Cancer. 2010; 127:1650–1659. PMID: 20073066.

15. Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006; 98:1819–1825. PMID: 17179483.

16. Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007; 16:2213–2217. PMID: 17971518.

17. Scher HI, Eisenberger M, D'Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004; 22:537–556. PMID: 14752077.

18. Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010; 116:3389–3398. PMID: 20586112.
19. Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005; 162:318–325. PMID: 16014776.

20. Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ. Is statin use associated with prostate cancer aggressiveness? BJU Int. 2010; 105:1222–1225. PMID: 19888973.

21. Roy M, Kung HJ, Ghosh PM. Statins and prostate cancer: role of cholesterol inhibition vs. prevention of small GTPbinding proteins. Am J Cancer Res. 2011; 1:542–561. PMID: 21984972.
22. Schaffner CP. Prostatic cholesterol metabolism: regulation and alteration. Prog Clin Biol Res. 1981; 75:279–324. PMID: 6175978.
23. Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim Biophys Acta. 2013; 1835:219–229. PMID: 23357067.

24. Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010; 28:2653–2659. PMID: 20421534.

Table 1
Baseline characteristics of the patients
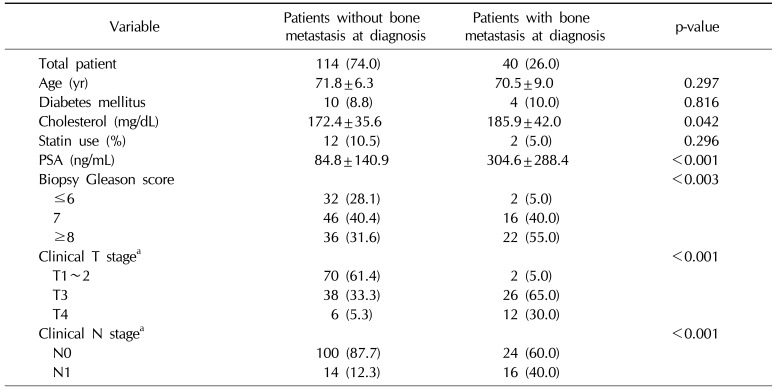
Table 2
Multivariate model for time to PSA progression according to age and clinical variables
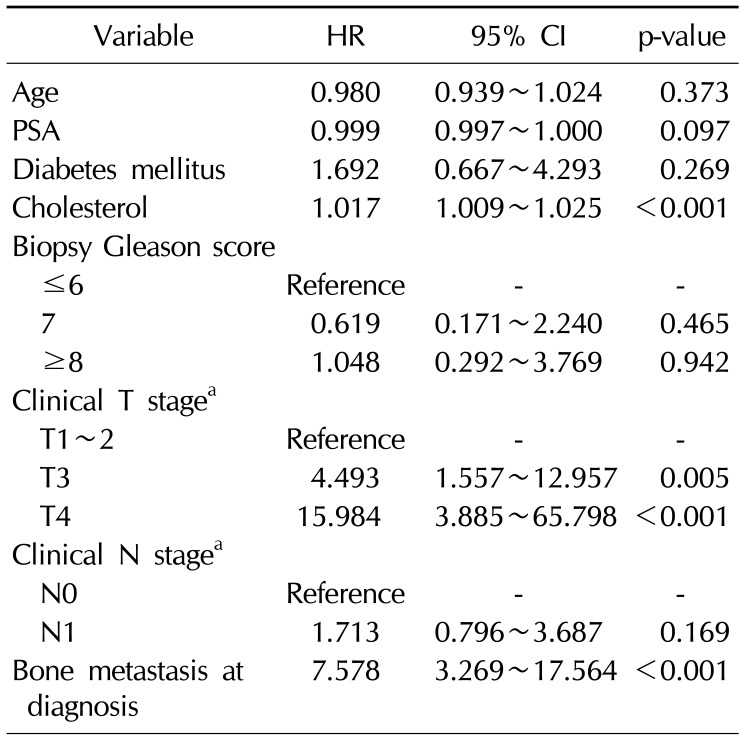
Table 3
Multivariate model for time to PSA progression according to age and clinical variables in patients with bone metastasis at diagnosis
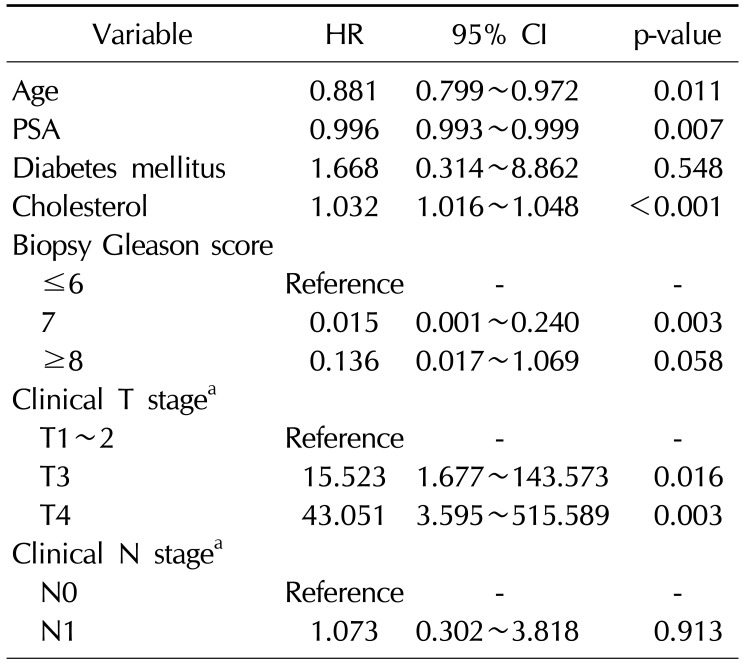
Table 4
Multivariate model for time to PSA progression according to age and clinical variables in patients without bone metastasis at diagnosis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download