Abstract
Purpose
The present study was conducted to evaluate the favorable or harmful effects of betulinic acid (BA) on a diabetic reproductive system.
Materials and Methods
In this experimental study, 60 male Naval Medical Research Institute mice (20∼25 g) were randomly divided into 6 groups: control, diabetes, diabetes+BA (10, 20, and 40 mg/kg), and diabetes+ metformin (200 mg/kg). A diabetic model was induced by a single dose of streptozotocin (STZ) (65 mg/kg) injection intraperitoneally 15 minutes after an intraperitoneal administration of nicotinamide (NA) (120 mg/kg). BA and metformin were gavaged for 2 weeks after confirmed diabetes induction in the treatment groups. One day after the last treatment, plasma luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone levels were evaluated. The cauda epididymis and testis were removed to analyze the sperm count and testis histopathology.
Results
LH levels increased in diabetic (p<0.001) and diabetic BA-treated mice (p=0.009). Plasma levels of testosterone (p< 0.001) and sperm count (p=0.04) decreased in these groups when compared to the control group. Furthermore, administration of 10 mg/kg (p=0.001), 20 mg/kg (p=0.004), or 40 mg/kg (p<0.001) of BA led to a greater reduction in plasma testosterone levels compared to the diabetes group. Seminiferous tubule vacuole numbers increased in diabetic and diabetic BA-treated mice, but testis morphology and FSH level assessment revealed no significant differences between the groups.
The incidence of diabetes mellitus (DM) is rising rapidly worldwide; it is estimated that 366 million people will be living with DM by 2030 [1]. This disease can induce long-term damage and dysfunction in various organs, including nephropathy leading to renal failure, cardiovascular symptoms, and testicular impairment leading to sexual dysfunction. Glucose metabolism plays an important role in spermatogenesis, and several studies in both animals and humans have confirmed the deleterious effect of hyperglycemia on sexual function, including semen parameters [1]. There are serious concerns that DM can lead to a progressive deterioration of human reproductive health, specifically in younger patients with diabetes. Some of these concerns include a DM-induced decline in male sperm quality and influence on the hypothalamic–pituitary–gonadal (HPG) axis [2]. Previous studies have indicated that male hypogonadism is caused by a variety of chronic diseases, such as type 2 diabetes, and it is characterized by low levels of total testosterone and an increase in gonadotropins. These changes are related to a steroidogenesis defect in Leydig cells, as shown by both in vivo and in vitro studies [34]. Sexual organ disorders in diabetic cases can also result from oxidative stress and the imbalance between reactive oxygen species (ROS) generation and antioxidant enzyme activity [5].
3β-Hydroxylup-20(29)-en-28-oic acid, also known as betulinic acid (BA), is a pentacyclic lupane-type triterpene that is distributed throughout the Plantae kingdom. Many interesting biological effects of BA have been described, including anti-inflammatory, immunomodulatory, antiangiogenic, antifibrotic, and hepatoprotective effects [6]. BA can be extracted from various plants, including Quisqualis fructus, Coussarea paniculata, Caesalpinia paraguariensis, Vitex negundo, Berlinia grandiflora, Ziziphus joazeiro, Uapaca nitida, Ipomea pes-caprae, Ancistrocladus heyneanus, Diospyros leucomelas, and Syzygium claviforum [7]. Some of these plants have been used for treatment of DM via improved glucose tolerance, hepatic insulin resistance, and alterations of β-cell function and mass. It has been proposed that the anti-diabetic effects of these plants derive from the presence of BA in them; however, there are no studies about the effects of this agent on the reproductive system in a diabetic situation [8]. Therefore, working from the high prevalence of type 2 diabetes and the effect of BA on the treatment of diabetes, as well as the absence of studies concerning the favorable or harmful effects of this agent on the diabetic reproductive system, the present study was conducted to evaluate the effects of BA on the male reproductive system of a streptozotocin-nicotinamide (STZ-NA)-induced diabetic mouse model.
In this experimental study, 60 adult male Naval Medical Research Institute mice weighing 20∼25 g were obtained from the Ahvaz Jundishapur University of Medical Sciences (AJUMS) animal facility. Mice used in this study were treated in accordance with the principles and guidelines on animal care of AJUMS as reviewed by an ethics committee (IR.AJUMS.REC.1395.141), and kept at a 20℃±4℃ temperature with a 12-hour light/dark cycle. They had access to tap water and commercial chow ad libitum.
After a one-week period of animal acclimatization, a single dose of 65 mg/kg of STZ (dissolved in a citrate buffer, pH 4.5; Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally 15 minutes after an intraperitoneal administration of 120 mg/kg of NA (dissolved in normal saline; Sigma-Aldrich) for the induction of type 2 diabetes [9]. The presence of DM was confirmed by assaying blood glucose levels at 1 week after NA-STZ injection. Mice with a blood glucose level more than 200 mg/dL were used in subsequent experiments [10]. Thereafter, BA (Sigma-Aldrich) and metformin (as a standard type 2 diabetes drug; Sigma-Aldrich) were orally administered by stainless steel feeding tubes (24 gauge [0.6 mm outer diameter×0.4 mm inner diameter]×25 mm) for 2 weeks after confirmed diabetes induction in the treatment groups. Hence, mice were divided into 6 groups (n=10): control, diabetes, diabetes+BA at 10 mg/kg, 20 mg/kg, and 40 mg/kg [11], and diabetes+metformin at 200 mg/kg [12].
Twenty-four hours after the last drug administration, the mice were anesthetized by ether and plasma samples were taken by cardiac puncture blood collection and centrifuging at 3,500 rpm for 20 minutes, and kept at −80℃ until hormonal measurements were performed. The right testes of all animals were removed immediately and testicular weight, width, length were assessed in each group. The volume of the testis was analyzed by using the following formula: volume=(D2/4×π ) L×K (length [L], width [D], K=0.9, π =3.14) [13].
The cauda epididymis of every mouse was separated and transferred into a Petri dish containing 3 mL 0.9% normal saline and minced into small pieces for sperm counting. Then, spermatozoa were vented into surrounding fluid after squeezing these slices. In order to estimate the sperm count, a drop of Petri dish solution was transferred into a Neubauer chamber (depth 0.100 mm and area 0.0025 mm2; HBG Henneberg-Sander GmbH, Gießen, Germany). Sperm counting was assayed manually in white blood cell chambers under light microscopy (Olympus Light Microscope; Olympus Corp., Tokyo, Japan). Finally, data were expressed as the number of sperm per milliliter [14].
Hormonal assessment was performed for plasma follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone levels by enzyme-linked immunosorbent assay with commercial assay kits (DRG Instruments GmbH, Marburg, Germany). The hormone detection sensitivity per assay tube of each kit was 1.27 mIU/mL for FSH, 0.856 mIU/mL for LH, and 0.287 nmol/L for testosterone.
After blood collection, the left testes of the animals were removed and fixed in 10% formalin solution. Then, they were dehydrated in graded alcohol concentrations and embedded in paraffin. Sections of 5∼7 µm were prepared and stained with hematoxylin/eosin. Six microscopy slides were examined for assessment of histological changes in each mouse. For each slide, the mean of 6 fields was calculated. Diameters of the seminiferous tubules and the lumen diameter were measured by using Motic Images Plus 2.0 image analysis software (Motic, Hong Kong, China). The height of the seminiferous epithelium was calculated by subtracting the lumen diameter from the tubule diameter. For each animal, 150 tubules were analyzed. Evaluators were blinded to the treatment groups [15].
Data were statistically analyzed using SPSS Statistics ver. 15 (SPSS Inc., Chicago, IL, USA) as mean±standard error of mean with one-way analysis of variance, followed by post hoc least significant difference tests. Differences were considered statistically significant at p<0.05.
The results of the testis morphology assessment presented here indicate that there were no significant differences in testis weight, length, width, or volume between groups, though there was a tendency toward a decrease in testicular weight and volume after BA treatment (p>0.05) (Table 1).
As shown in Fig. 1, plasma LH levels increased in untreated diabetic mice (p<0.001), diabetic mice treated with 10 mg/kg (p=0.002), 20 mg/kg (p=0.009), and 40 mg/kg (p=0.009) of BA, and metformin-treated (p=0.009) mice compared to the control group. Further, metformin treatment showed a significant increase in plasma LH level versus the untreated diabetic group (p=0.03). Plasma level measurement of FSH indicated that there were no significant changes among the groups after the induction and treatment of diabetes (Fig. 2). Treatment with BA or metformin revealed a significant decrease in plasma levels of testosterone in comparison with the control group (p<0.001). Further, administration of 10 mg/kg (p=0.001), 20 mg/kg (p=0.004), and 40 mg/kg (p<0.001) of BA and metformin (p=0.01) led to a greater reduction in plasma testosterone levels compared to the untreated diabetes group (Fig. 3).
The results showed that epididymal sperm counts were significantly reduced in untreated diabetic (p=0.04) and diabetic animals treated with 10 mg/kg (p=0.02), 20 mg/kg (p=0.03), 40 mg/kg (p=0.03) of BA compared to the control group. However, there were no significant differences in the sperm counts of diabetic metformin-treated mice compared to control and diabetes groups (p=0.27) (Fig. 4).
Histological assessment indicates that the diameters of seminiferous tubules and the epithelium decreased in diabetic mice treated with 10 mg/kg (p=0.04), 20 mg/kg (p=0.03), 40 mg/kg (p=0.007) of BA when compared to the control group. Furthermore, the same effects were observed in diabetes+BA 20 mg/kg and 40 mg/kg groups compared with the untreated diabetes group (p=0.03 and p=0.009 respectively) (Table 2). The appearance of testis histology was normal in the control group. Many vacuoles were observed in the seminiferous tubule epithelia of diabetic mice and the number and size of these vacuoles increased in the BA-treated diabetic groups in a dose-dependent manner. In addition, these changes showed a tendency to improve after metformin administration in diabetic mice (Fig. 5).
The present results indicate that induction of type 2 diabetes and BA administration did not change testis morphology. High blood glucose levels might cause abnormalities in homeostatic regulation, and the anterior pituitary gland, as a sensitive target, may be affected by hyperglycemia, resulting in a malfunction of the pituitary-gonadal axis. This phenomenon can destroy germ cells and decrease the size of the seminiferous tubules and seminal vesicles, but the lack of alteration in testicular weight observed here might be because testes are composed of many kinds of tissue and the destruction of some seminiferous tubules might not affect the total weight of the testes [16]. In addition, the dosage or duration of BA may not have been sufficient to produce significant effects in testis weight and volume. A long-term administration period may produce greater effects. Abnormal glucose homeostasis and hyperglycemia have adverse outcomes on testicular function and spermatogenesis in type 2 diabetic male cases [1]. Indeed, the findings of the present study revealed that STZ-NA-induced diabetes can produce male reproductive dysfunction through changes to plasma testosterone levels and sperm count reduction. Furthermore, plasma levels of LH increased to compensate for these type 2 diabetic model reductions. Primary hypogonadism or hypergonadotropic hypogonadism can occur with a testicular origin and is characterized by low testosterone and high LH levels [17]. Consistent with our results, Asare-Anane et al [18] showed an increase in LH and decrease in testosterone levels concomitant with an insignificant difference in FSH level between type 2 diabetic and non-diabetic patients that indicated that primary hypogonadism had occurred in these cases. Thus, the present findings could suggest STZ-NA-induced primary hypogonadism along with DM.
Insulin intolerance and hyperglycemia or its metabolites caused by type 2 diabetes have been associated with disruption of the HPG axis, leading to imbalances in the levels of sex steroid hormones, including a decrease in testosterone secretion by Leydig cells. Leydig cell secretion of testosterone is necessary for spermatogenesis and fertility. This hormone can act directly on target cells through its androgen receptors. Insulin resistance related to diabetes is associated with impairments of Leydig cell testosterone secretion and receptors due to stress induced at the testicular level [19]. The whole body metabolism, including endocrine disruption, testicular energy consumption, and male reproductive function, is disrupted by DM. Sertoli cells provide a nutritional supporting environment for a heterogeneous population of somatic and germ cells in the testes, which could be altered by DM. Furthermore, it has been noted that altered metabolism within the testicular environment is associated with male reproductive insufficiencies [20]. Therefore, it could be suggested that the present hormonal assessment findings may be the result of an imbalance of testicular energy consumption in type 2 diabetic animals. The relationship between male infertility and DM has been discussed in some experiments conducted on STZ-induced diabetic models. It has been indicated that excess production of ROS and disruption in antioxidant defenses is more likely to occur in a hyperglycemic state. Enhancement of ROS generation through hyperglycemia can cause the oxidation of proteins and lipids and damage to macromolecules such as DNA [21]. Furthermore, the membranes of spermatozoa are rich in polyunsaturated fatty acids and very susceptible to oxidative stress or free radicals [22]. The study of Butchi Akondi et al [21] demonstrated that diabetic mice showed a significant reduction in sperm parameters like sperm count and motility through an increase in oxidative stress and ROS production. Hence, the present study suggests that diabetes-induced oxidative stress might be one of the main mechanisms of sperm count reduction; however, further studies are required to determine the exact mechanism of this phenomenon.
One study has indicated that spermatozoal mitochondria are extremely susceptible to anticancer agents such as BA, and it has been suggested that their side effects on the male reproductive system should be considered [23]. Furthermore, there are many herbs with proposed anti-diabetic effects, including V. negundo, and I. pes-caprae that exhibit noteworthy hypoglycemic activity, restoring the altered serum levels of hepatic enzymes and engaging in hepatoprotective activities. Moreover, it has been suggest that the anti-diabetic effects of these plants can be attributed to the presence of BA in them [2425]. Hence, according to the reducing effect that all doses of BA had on plasma testosterone levels, sperm counts, and the deterioration of primary hypogonadism in diabetic mice, it could be suggested that more caution should be taken in the utilization of the abovementioned anti-diabetic plants and BA, either in low or high doses, for the treatment of chronic diseases such as diabetes and cancer. Future studies are required to reveal the exact safe anti-diabetic dose of BA. Consistent with the more toxic effects of low and high doses of BA, the study of Al-Yahya [26] suggested that this compound could behave as both an antioxidant and pro-oxidant, depending on concentration and free radical source.
Low intratesticular production of testosterone and consequent weak testosterone secretion into the serum have been shown to be insufficient to induce virilization and maintenance, but were nevertheless sufficient to produce complete spermatogenesis and sperm production in men [27]. Hence, it could be suggested that BA acts through the mentioned mechanisms to produce similar sperm numbers to the untreated diabetes group despite lower levels of testosterone production.
Vacuolation is one of the most common symptoms of Sertoli cell injury. Sertoli cell cytoplasm shows swelling at the basal cytoplasm before vacuolation and, subsequent to vacuolation, germ cell degeneration or disorganization is generally seen. Thus, these events may lead to rapid degeneration of spermatocytes and a decrease in sperm count and fertility parameters [28]. Furthermore, one of the main functions of the Sertoli cell is to produce substances for germ cell survival and provide an essential environment for spermatogenesis. Induction of diabetes is associated with an increase in the number and size of vacuoles in Sertoli cell cytoplasm in the testicular tissue and this vacuolization may alter spermatogenesis with lower spermatozoa numbers [29]. Therefore, consistent with previous studies, the present histopathological results suggest vacuolization in the seminiferous tubule epithelium of diabetic BA-treated mice with impaired Sertoli cell function and reduced sperm count. Testosterone is delivered to the epididymis through the seminiferous tubular fluid produced by Sertoli cells. Any compound causing decreased testicular testosterone results in decreased seminiferous tubular diameter, atrophy of the luminal epithelium, or vacuolization in Sertoli cells [30]. Hence, it could be that administration of BA induced these effects rather than alleviating diabetic alteration via reduced testosterone levels.
In conclusion, this study indicated that STZ-NA can induce diabetic alterations in the male reproductive system, including decrease in testosterone and sperm count and increase in plasma LH levels and seminiferous tubule vacuoles. Furthermore, diabetic BA-treated mice demonstrated a worse situation, with a greater reduction in plasma testosterone level and an increase in testicular vacuolization. Thus our findings suggest that the administration of anti-diabetic plants containing BA for the treatment of diabetes be pursued with greater caution.
Figures and Tables
Fig. 1
The effect of BA on plasma LH levels of normal and streptozotocin-nicotinamide-induced diabetic mice. Values are presented as mean±standard error, n=10. LH: luteinizing hormone, DM: diabetes mellitus, BA: betulinic acid (mg/kg), Met: metformin. *p<0.05 compared with the DM group, ##p<0.01, ###p<0.001 compared with the control group.
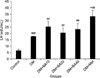
Fig. 2
The effect of BA on plasma FSH levels of normal and streptozotocin-nicotinamide-induced diabetic mice. Values are presented as mean±standard error, n=10. FSH: follicle stimulating hormone, DM: diabetes mellitus, BA: betulinic acid (mg/kg), Met: metformin.
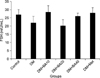
Fig. 3
The effect of BA on serum testosterone levels of normal and streptozotocin-nicotinamide-induced diabetic mice. Values are presented as mean±standard error, n=10. DM: diabetes mellitus, BA: betulinic acid (mg/kg), Met: metformin. **p<0.01, ***p<0.001 compared with the DM group, ###p<0.001 compared with the control group.
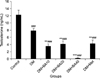
Fig. 4
Effect of BA on the sperm count of normal and streptozotocin-nicotinamide-induced diabetic mice. Values are presented as mean±standard error, n=10. DM: diabetes mellitus, BA: betulinic acid (mg/kg), Met: metformin. #p<0.05 compared with the control group.
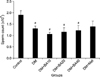
Fig. 5
The effect of betulinic acid (BA) on testis histopathology (H&E, ×400). (A) Control. (B) Diabetes. (C) Diabetes+10 mg/kg of BA. (D) Diabetes+20 mg/kg of BA. (E) Diabetes+40 mg/kg of BA. (F) Diabetes+metformin. V: vacuolation.
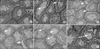
Table 1
The effect of BA on the testicular morphology of normal and diabetic mice

Table 2
The effect of BA on the testicular seminiferous tubules and epithelium of normal and diabetic mice

ACKNOWLEDGEMENTS
This study was labeled Student Research Project No. 94S134, and was supported financially by the Student Research Committee of Ahvaz Jundishapur Medical Sciences University, Ahvaz, Iran.
References
1. Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 2015; 17:948–953.

2. Mulholland J, Mallidis C, Agbaje I, McClure N. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod Biomed Online. 2011; 22:215–219.

3. Zheng R, Cao L, Cao W, Chu X, Hu Y, Zhang H, et al. Risk factors for hypogonadism in male patients with type 2 diabetes. J Diabetes Res. 2016; 2016:5162167.

4. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012; 33:145–153.

5. Ahangarpour A, Oroojan AA, Heidari H, Ehsan G, Rashidi Nooshabadi MR. Effects of hydro-alcoholic extract of Rhus coriaria (Sumac) seeds on reproductive complications of nicotinamide-streptozotocin induced type-2 diabetes in male mice. World J Mens Health. 2014; 32:151–158.
6. Csuk R. Betulinic acid and its derivatives: a patent review (2008-2013). Expert Opin Ther Pat. 2014; 24:913–923.
7. Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005; 12:657–666.

8. Ko BS, Kang S, Moon BR, Ryuk JA, Park S. A 70% ethanol extract of mistletoe rich in Betulin, betulinic acid, and Oleanolic acid potentiated β-cell function and mass and enhanced hepatic insulin sensitivity. Evid Based Complement Alternat Med. 2016; 2016:7836823.
9. Ahangarpour A, Oroojan AA, Heydari H. Effect of hydro-alcoholic extract of dorema aucheri on serum levels of testosterone, FSH and sperm count in nicotinamide-STZ-induced diabetic rat models. ZUMS J. 2013; 21:22–31.
10. Khanra R, Dewanjee S, K Dua T, Sahu R, Gangopadhyay M, De Feo V, et al. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med. 2015; 13:6.

11. Oyebanji BO, Saba AB, Oridupa OA. Studies on the anti-inflammatory, analgesic and antipyrexic activities of betulinic acid derived from Tetracera potatoria. Afr J Tradit Complement Altern Med. 2013; 11:30–33.

12. Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of type 2 diabetic embryopathy. Diabetes. 2015; 64:2526–2536.

13. Ahangarpour A, Oroojan AA, Heidari H. Effects of exendin-4 on male reproductive parameters of d-galactose induced aging mouse model. World J Mens Health. 2014; 32:176–183.

14. Ahangarpour A, Oroojan AA, Aliakbari FR. Effects of C-peptide and nicotinamide on serum LH, FSH, testosterone levels and sperm count in nicotinamide/streptozotocin-induced-diabetes in mice. Acta Endo (Buc). 2014; 10:588–594.
15. Talebi AR, Khorsandi L, Moridian M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet. 2013; 30:1203–1209.

16. Aritajat S, Wutteerapol S, Saenphet K. Anti-diabetic effect of Thunbergia laurifolia Linn. aqueous extract. Southeast Asian J Trop Med Public Health. 2004; 35:Suppl 2. 53–58.
17. Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol. 2004; 6:Suppl 6. S3–S8.
18. Asare-Anane H, Ofori EK, Yeboah FA, Tagoe EA, Bani SB, Bawah AT, et al. Primary hypogonadism in Ghanaian men with type 2 diabetes mellitus. Int J Sci Technol Res. 2013; 2:310–315.
19. Liu CY, Hsu YJ, Chien YW, Cha TL, Tsao CW. Dietary resistant maltodextrin ameliorates testicular function and spermatogenesis in streptozotocin-nicotinamide-induced diabetic rats. Andrologia. 2016; 48:363–373.

20. Rato L, Alves MG, Dias TR, Cavaco JE, Oliveira PF. Testicular metabolic reprogramming in neonatal streptozotocin-induced type 2 diabetic rats impairs glycolytic flux and promotes glycogen synthesis. J Diabetes Res. 2015; 2015:973142.

21. Butchi Akondi R, Kumar P, Annapurna A, Pujari M. Protective effect of rutin and naringin on Sperm Quality in Streptozotocin (STZ) induced type 1 diabetic rats. Iran J Pharm Res. 2011; 10:585–596.
22. Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med. 2014; 11:1–8.

23. Dathe S, Paasch U, Grunewald S, Glander HJ. Mitochondrial damage in human sperm caused by the antineoplastic agent betulinic acid. Hautarzt. 2005; 56:768–772.
24. Prasanna Raja P, Sivakumar V, Riyazullah MS. Antidiabetic potential of aqueous and ethanol leaf extracts of vitex negundo. Int J Pharmacogn Phytochem Res. 2012; 4:38–40.
25. Suhasini S, Elanchezhiyan C, Babby A. Hepatoprotective effect of Ipomoea pes-caprae leaves extract in streptozotocin induced diabetic rats. Int J Pharm Pharm Sci Res. 2014; 4:27–30.
26. Al-Yahya AA. Reproductive toxicity of orthosiphon stamineus Benth (java tea) in Swiss albino mice. Br J Pharmacol Toxicol. 2013; 4:181–187.
27. Achard C, Courtillot C, Lahuna O, Méduri G, Soufir JC, Lière P, et al. Normal spermatogenesis in a man with mutant luteinizing hormone. N Engl J Med. 2009; 361:1856–1863.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download