Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Changes in clinical parameters at 8 months after intervention with either testosterone replacement therapy (TRT group, n=54) or placebo (control group, n=52) in patients with testosterone deficiency syndrome. At 8 months after intervention, the improvement in total testosterone levels, IIEF-5 score, AMS score, and BDI score was significantly higher (*) in the TRT group than in the control group. *p<0.05 compared with control group. Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination. |
 | Fig. 2Overview of K-MMSE scores for patients with testosterone deficiency syndrome, stratified according to whether they received testosterone replacement therapy (TRT group, n=13) or a placebo (control group, n=12). Only data for patients with cognitive impairment at baseline (K-MMSE score <25) are given. At 8 months after intervention, the K-MMSE scores improved significantly (*) in the TRT group but not in the control group. *p<0.05 compared with baseline values. K-MMSE: Korean Mini-Mental State Examination. |
Table 1
Baseline characteristics of patients with testosterone deficiency syndrome
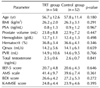
Values are presented as mean±standard deviation.
TRT: testosterone replacement therapy, BMI: body mass index, PSA: prostate-specific antigen, Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.
Table 2
Clinical parameters of patients with testosterone deficiency syndrome who received TRT (n=54)
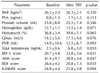
Values are presented as mean±standard deviation.
TRT: testosterone replacement therapy, BMI: body mass index, PSA: prostate-specific antigen, Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.
Table 3
Clinical parameters of patients with testosterone deficiency syndrome who did not undergo testosterone replacement therapy (control group, n=52)
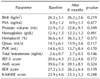
BMI: body mass index, PSA: prostate-specific antigen, Qmax: maximal urinary flow rate, PVR: postvoid residual volume, IIEF-5: 5-item version of the International Index of Erectile Function questionnaire, AMS: Aging Males' Symptoms, BDI: Beck Depression Inventory, K-MMSE: Korean Mini-Mental State Examination.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download