Abstract
Purpose
The aim of this study was to investigate the anti-inflammatory and anti-oxidative effects of a multi-herbal formula known as WSY-1075 in the treatment of chronic bacterial prostatitis in a rat model.
Materials and Methods
Experimental chronic bacterial prostatitis was induced in 32 Wistar rats by instillation of a bacterial suspension (Escherichia coli, 108 colony-forming units [CFU]/mL) into the prostatic urethra. After the induction of prostatitis, the rats were randomly divided into one of 4 treatment groups: control (n=8), ciprofloxacin (n=8), WSY-1075 (400 mg/kg) (n=8), and WSY-1075 (400 mg/kg)+ciprofloxacin (n=8). After 4 weeks of treatment, microbiological data from prostate tissue cultures, level of prostatic pro-inflammatory cytokines (tumor necrosis factor-α [TNF-α], interleukin [IL]-6, and IL-8), anti-oxidant effects (superoxide dismutase [SOD]), and histological findings were noted.
Results
The WSY-1075, ciprofloxacin, and WSY-1075+ciprofloxacin groups showed fewer CFUs in prostate tissue cultures than the control group. The WSY-1075, ciprofloxacin and WSY-1075+ciprofloxacin groups showed statistically significantly lower levels of the pro-inflammatory cytokines TNF-α, IL-6, and IL-8 than the control group. SOD levels in the WSY-1075, ciprofloxacin and WSY-1075+ciprofloxacin groups were significantly higher than in the control group.
Chronic bacterial prostatitis is the most common cause of recurrent urinary tract infections in males. It is known that up to half of all men suffer from symptoms of prostatitis at some point in their lives. Chronic bacterial prostatitis is characterized by recurrent urinary tract infections and the persistence of pathogenic bacteria in the prostatic fluid [1]. In general, fluoroquinolone is recommended as the drug of choice because it covers the bacterial spectrum of chronic bacterial prostatitis and penetrates best into the prostatic compartments. However, its long-term efficacy is poor because bacterial pathogens are protected from antibiotics by aggregating in microcolonies or biofilms localized to the affected prostatic duct [23]. Chronic bacterial prostatitis is also treated using α-blockers and anti-inflammatory agents,but no definitive cure exists.
Alternative medical therapies and phytotherapies are gaining popularity, and these treatments are often first-line therapies for various chronic medical conditions in Europe and Asia. Cernilton (bee pollen extract), quercetin, cranberry juice, and zinc are typical examples [4].
Evidence increasingly indicates that inflammation plays an important role in chronic prostatitis (CP) [5]. Thus, levels of proinflammatory cytokines are associated with prostatitis. Herbal formulas such as Corni fructus, Angelica gigantis radix, Lycii fructus, Cervi parvum cornu, Ginseng radix rubra, and Cassiae cortkex are widely known nutrient tonics in the general population. Their extracts, individually or in combination, have been shown to have anti-inflammatory effects [678910]. The aim of this study was to investigate the anti-inflammatory and anti-oxidative effects of a multi-herbal formula known as WSY-1075 in the treatment of chronic bacterial prostatitis in a rat model.
The herbal formula used in our study was WSY-1075. The major ingredients of WSY-1075 were obtained from 6 plants: 25% C. fructus, 25% A. gigantis radix, 25% L. fructus, 10% C. parvum cornu, 10% G. radix rubra, and 5% C. cortkex. A mixture of the dried seeds of the 6 plants was extracted with tap water (0.25 g/mL) for 3 hours by boiling. The extracts were filtered and concentrated in vacuo and then lyophilized. A venture company, Korea Bio Medical Science Institute Co. Ltd., Andong, Korea, which is developing oriental herbal medicines, developed this product as a health supplement.
The experimental animals (12-week-old male Wistar rats) were purchased from Samtaco Bio, Co. (Osan, Korea). The rats were housed in an animal room maintained at a constant temperature and humidity with a 12-hour light/dark cycle. The treatment protocols were approved by the Institutional Animal Care and Use Committee (CUMC-2015-0155-01) and handled according to National Institute of Health (NIH) guidelines.
Thirty-two male Wistar rats were used in the study. Experimental chronic bacterial prostatitis was induced by the instillation of a bacterial suspension (Escherichia coli, 108 colony-forming units [CFU]/mL) into the prostatic urethra. Previous studies have suggested that intraurethral inoculation is the best method of producing bacterial prostatitis in rats [11].
The experimental animals were anesthetized with ether, the genital area was sterilized with 70% alcohol, a sterile polyethylene tube (outer diameter of 0.9 mm, 2.5 cm long) was inserted into the urethra, and 0.2 mL of an E. coli Z17 suspension (O2:K1:H−, 108 CFU/mL) [12] was injected into the prostatic urethra using insulin syringes. Sufficient time for the bacteria to infiltrate to the inside of the prostate was ensured by preventing the excretion of urine through maintenance of anesthesia for 1 hour.
Four weeks after the induction of bacterial prostatitis, prostate tissue culturing was performed and the presence of E. coli was confirmed. The rats were randomly divided into one of 4 treatment groups: control (phosphate buffered saline [PBS]) (n=8), ciprofloxacin (n=8), WSY-1075 (400 mg/kg) (n=8), and WSY-1075 (400 mg/kg)+ciprofloxacin (n=8). The rats received the treatments through an orogastric tube once a day for 4 weeks. The PBS group was administered 1 g of PBS, and 2.5 mg/kg of ciprofloxacin diluted in 1 mL distilled water was administered to the ciprofloxacin groups once per day. After treatment, all rats were anesthetized using ketamine (50 mg/kg) and xylazine (12 mg/kg). The prostate was excised and the prostate capsule was meticulously removed in order to obtain only the prostate glands.
To investigate anti-inflammatory effects, levels of the tumor necrosis factor [TNF]-α, interleukin [IL]-6 and IL-8 cytokines were analyzed using enzyme-linked immunosorbent assays (ELISA). Blood obtained before sacrifice was centrifuged for 10 minutes (3,000 rpm, 4℃), and the supernatant was immediately transferred to a tube. The cytokine concentration was measured every 5 minutes for 30 minutes, using a spectrophotometer at 450 nm with an immunoassay ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
Superoxide dismutase (SOD) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide radical into either ordinary molecular oxygen or hydrogen peroxide. SOD activity (CuZnSOD and Mn SOD) was measured using the SOD Assay Kit-WST (Dojindo, Kumamoto, Japan), monitoring the decrease in the rate of superoxide-mediated reduction of nitroblue tetrazolium at 450 nm using a spectrophotometer. Malondialdehyde (MDA) assessment of the peroxidation reaction products generated by free radicals was performed with the double-heating method described by Draper and Hadley [13]. The method is based on the principle of producing maximum absorbance at a 532-nm wavelength by reacting MDA, which is the final product in fatty acid peroxidation with thiobarbituric acid [13].
Significant differences were found in all 3 treatment groups compared to the control group (p<0.05). Both the ciprofloxacin group and the WSY-1075 group showed significantly fewer CFUs than the control group. The WSY-1075+ciprofloxacin group showed the fewest CFUs (Table 1).
To investigate the effects of WSY-1075 on chronic bacterial prostatitis, levels of the inflammatory cytokines TNF-α, IL-6, and IL-8 were analyzed using. The WSY-1075 group, ciprofloxacin group, and WSY-1075+ciprofloxacin group showed significantly lower pro-inflammatory cytokine TNF-α levels than the control (PBS) group (69.71 pg/mL, 50.28 pg/mL, and 34.47 pg/mL vs. 100.13 pg/mL) (p=0.028, p=0.028, p=0.09, respectively). Moreover, the WSY-1075+ciprofloxacin group showed significantly lower TNF-α levels than the ciprofloxacin group (p=0.028) (Fig. 1). Levels of the pro-inflammatory cytokine IL-6 in the WSY-1075, ciprofloxacin, and WSY-1075+ ciprofloxacin groups were significantly lower than in the control group (32.32 pg/mL, 22.15 pg/mL, and 14.24 pg/mL, respectively, vs. 45.00 pg/mL) (p=0.028, p=0.009, p=0.009, respectively). Additionally, the WSY-1075+ciprofloxacin group had significantly lower IL-6 levels than the ciprofloxacin group (p=0.047) (Fig. 2). Levels of the pro-inflammatory cytokine IL-8 in the WSY-1075, ciprofloxacins and WSY-1075+ciprofloxacin group were significantly lower than in the control group (45.96 pg/mL, 32.13 pg/mL, and 20.7 pg/mL, respectively, vs. 60.05 pg/mL) (p=0.047, p=0.009, and p=0.009, respectively). Additionally, the WSY-1075+ciprofloxacin group showed significantly lower IL-8 levels than the ciprofloxacin group (p=0.047) (Fig. 3).
The SOD levels of the WSY-1075, ciprofloxacin, and WSY-1075+ciprofloxacin groups were significantly higher than in the control group (40.92 µg/mL, 45.67 µg/mL, and 59.85 µg/mL, respectively, vs. 25.68 µg/mL) (p=0.076, p=0.076, p=0.009). Additionally, the WSY-1075+ciprofloxacin group showed significantly higher SOD levels than the ciprofloxacin group (p=0.028) (Fig. 4).
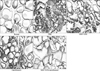
The control group showed extensive infiltration of inflammatory cells, including neutrophils, lymphocytes, and macrophages, as well as degeneration of glandular epithelial cells, suggesting the presence of bacterial prostatitis. In the ciprofloxacin-treated group and the WSY-1075-treated group, fewer inflammatory cells were found in the lumina, and the epithelial cells of the gland were improved compared to the control group. The WSY-1075+ciprofloxaxin group showed a nearly normal appearance of the glandular epithelium, with less infiltration of inflammatory cells (Fig. 5).
The pathophysiology of human bacterial prostatitis is thought to be associated with retrograde bacterial ascent from infected urine secondary to some form of intraprostatic ductal reflux. We consider the reproducible rat model used in this study to have many similarities to the natural history of human chronic bacterial prostatitis. This experimental animal model has been recognized as a chronic bacterial prostatitis animal model by many previous authors [3].
In this study, we demonstrated that WSY-1075 had an antimicrobial effect, an anti-inflammatory effect, and an antioxidative effect. All these results had a synergic effect when used with ciprofloxacin. WSY-1075 is composed of C. fructus, A. gigantis radix, L. fructus, C. parvum cornu, G. radix rubra, and C. cortkex [89141516].
In the 1990s, the NIH established a classification system for prostatitis that comprises infectious forms (acute and chronic), chronic pelvic pain syndrome (CPPS), and asymptomatic prostatitis [17]. Approximately 10% of men who suffer an episode of acute bacterial prostatitis go on to suffer chronic bacterial prostatitis, and similarly, 10% progress to CP/CPPS [18]. CPPS accounts for most of the prostatitis-like symptoms. The symptoms experienced by men with chronic bacterial prostatitis do not necessarily differ from those with CP/CPPS [19]. The etiology of CP/CPPS has not been conclusively elucidated, and non-curative but efficacious treatments exist. Antibiotics including fluoroquinolones, α-blockers and anti-inflammatory agents are used as first-line and second-line therapies. Third-line therapies include 5α-reductase inhibitors, saw palmetto, quercetin, tricyclic antidepressants, phosphodiesterase inhibitors, and glycosaminoglycans, but the evidence for their efficacy is only level III~IV [20]. Alternative medical therapies and phytotherapies are gaining popularity and these treatments are often first-line therapies for various chronic medical conditions in Europe and Asia. Quercetin has anti-inflammatory and anti-oxidant mechanisms. Cranberry juice reduces bacterial adherence, and zinc has an anti-microbial mechanism [4].
Evidence increasingly suggests that inflammation plays an important role in CP. [5]. Inflammation can lead to chronic prostatic diseases, such as CP and benign prostatic hyperplasia (BPH) [2122]. Therefore, pro-inflammatory cytokines are closely related to chronic prostatic diseases. IL-6 and IL-8 concentrations are elevated in CP patients, and IL-8 also may be a key mediator of CP/CPPS and BPH [23]. We suggest that the anti-inflammatory effect of WSY-1075 can be helpful for not only chronic bacterial prostatitis, but also other forms of CP/CPPS and BPH. Yoon et al [24] have demonstrated that WSY-1075 had anti-inflammatory effects in a non-bacterial prostatitis rat model.
WSY-1075 also showed an anti-oxidative effect in our study. Oxidative stress and oxidative damage are closely involved in the course of CP [25] and BPH. In CP, inflammatory reactions lead to the presence of many inflammatory cells and inflammatory mediators, causing abnormal metabolism of the hypoxanthine/xanthine oxidase system and the xanthine/xanthine oxidase system, producing many abnormal metabolites. These reactions also activate and release cyclooxygenase-2, transcription factor nuclear factor-kappa B and inflammatory oxidants. They release free radicals and reactive oxygen species. Excessive free radicals and reactive oxygen species can interact directly with and damage DNA [25]. Increased oxidative DNA damage is also present in BPH and plays a role in tissue proliferation [26].
We analyzed serum levels of anti-inflammatory cytokines and SOD, similarly to previous studies of WSY-1075 [2427]. This may be the principal limitation of this study. In subsequent research, we will analyze cytokines not only in the serum but also in the prostatic tissue. The small number of animals (n=8 in each group) is also a limitation of this study.
In this study, we demonstrated the effectiveness of WSY-1075 in a chronic bacterial prostatitis rat model. However, we additionally expect that WSY-1075 may be useful for all kinds of chronic prostatic diseases with mechanisms involving oxidative effects, including CP/CPPS.
The results of our study indicate that the herbal formula WSY-1075, which was prepared from a mixture of C. fructus, A. gigantis radix, L. fructus, C. parvum cornu, G. radix rubra, and C. cortkex, had anti-inflammatory and anti-oxidative effects in a chronic bacterial prostatitis rat model. We expect that WSY-1075 may be useful for the clinical treatment of chronic bacterial prostatitis.
Figures and Tables
Fig. 1
Effects of WSY-1075 and WSY-1075+ciprofloxacin on serum TNF-α levels. Each value represents the mean±standard deviation. TNF: tumor necrosis factor, PBS: phosphate-buffered saline. ap<0.05, compared with the control group; bp<0.01, compared with the control group; cp<0.05, compared with the ciprofloxacin group.
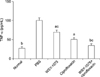
Fig. 2
Effects of WSY-1075 and WSY-1075+ciprofloxacin on serum IL-6 levels. Each value represents the mean±standard deviation. IL: interleukin, PBS: phosphate-buffered saline. ap<0.01, compared with the control group; bp<0.05, compared with the control group; cp<0.05, compared with the ciprofloxacin group.
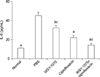
Fig. 3
Effects of WSY-1075 and WSY-1075+ciprofloxacin on serum IL-8 levels. Each value represents the mean±standard deviation. IL: interleukin, PBS: phosphate-buffered saline. ap<0.01, compared with the control group; bp<0.05, compared with the control group; cp<0.05, compared with the ciprofloxacin group.
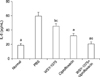
Fig. 4
Effects of WSY-1075 and WSY-1075+ciprofloxacin on serum SOD levels. Each value represents the mean±standard deviation. SOD: superoxide dismutase, PBS: phosphate-buffered saline. ap<0.05, compared with the control group; bp<0.01, compared with the control group; cp<0.05, compared with the WSY-1075+ciprofloxacin group.
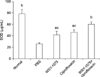
Fig. 5
Histopathologic findings of the prostate in each group (H&E, ×100). The ciprofloxacin group and the WSY-1075 group showed fewer inflammatory cells in the lumina, and improved epithelial cells in the gland compared to the PBS group. WSY-1075+ciprofloxaxin group showed a nearly normal appearance of the glandular epithelium with less infiltration of inflammatory cells. PBS: phosphate-buffered saline.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant HI15C0099).
References
1. Pfau A. Prostatitis. A continuing enigma. Urol Clin North Am. 1986; 13:695–715.
2. Wagenlehner FM, Weidner W, Naber KG. Therapy for prostatitis, with emphasis on bacterial prostatitis. Expert Opin Pharmacother. 2007; 8:1667–1674.

4. Shoskes DA, Manickam K. Herbal and complementary medicine in chronic prostatitis. World J Urol. 2003; 21:109–113.

5. Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, et al. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000; 164:214–218.
6. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, et al. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009; 58:431–436.

7. Park CH, Noh JS, Kim JH, Tanaka T, Zhao Q, Matsumoto K, et al. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol Pharm Bull. 2011; 34:1559–1565.

8. Lee JH, Lee JH, Lee YM, Kim PN, Jeong CS. Potential analgesic and anti-inflammatory activities of Panax ginseng head butanolic fraction in animals. Food Chem Toxicol. 2008; 46:3749–3752.

9. Oh YC, Cho WK, Im GY, Jeong YH, Hwang YH, Liang C, et al. Anti-inflammatory effect of Lycium Fruit water extract in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Int Immunopharmacol. 2012; 13:181–189.

10. Kim KW, Kim KS, Park SD, Kim JK, Chung KH, Kim DS, et al. Effect of Cervus korean TEMMINCK var. mantchuricus Swinhoe on protease activities, antioxidant and free radical damages in rheumatis arthritis rats. Toxicol In Vitro. 2008; 22:80–86.

11. Goto T, Kawahara M, Kawahara K, Mahinose S, Mizuma Y, Sakamoto N, et al. Experimental bacterial prostatitis in rats. Urol Res. 1991; 19:141–144.

12. Terai A, Yamamoto S, Mitsumori K, Okada Y, Kurazono H, Takeda Y, et al. Escherichia coli virulence factors and serotypes in acute bacterial prostatitis. Int J Urol. 1997; 4:289–294.

13. Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990; 186:421–431.
14. Jung JS, Shin JA, Park EM, Lee JE, Kang YS, Min SW, et al. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem. 2010; 115:1668–1680.

15. Sung YH, Chang HK, Kim SE, Kim YM, Seo JH, Shin MC, et al. Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells. J Med Food. 2009; 12:788–795.

16. Kim JH, Jeong JH, Jeon ST, Kim H, Ock J, Suk K, et al. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-kappaB activation in macrophages. Mol Pharmacol. 2006; 69:1783–1790.
17. Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999; 282:236–237.

18. Yoon BI, Kim S, Han DS, Ha US, Lee SJ, Kim HW, et al. Acute bacterial prostatitis: how to prevent and manage chronic infection? J Infect Chemother. 2012; 18:444–450.

19. Wagenlehner FM, Pilatz A, Bschleipfer T, Diemer T, Linn T, Meinhardt A, et al. Bacterial prostatitis. World J Urol. 2013; 31:711–716.

20. Murphy AB, Macejko A, Taylor A, Nadler RB. Chronic prostatitis: management strategies. Drugs. 2009; 69:71–84.
21. Sciarra A, Di Silverio F, Salciccia S, Autran Gomez AM, Gentilucci A, Gentile V. Inflammation and chronic prostatic diseases: evidence for a link? Eur Urol. 2007; 52:964–972.

22. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007; 51:1202–1216.

23. Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007; 51:524–533.

24. Yoon BI, Bae WJ, Kim SJ, Kim HS, Ha US, Sohn DW, et al. The anti-inflammatory effects of a new herbal formula (WSY-1075) in a nonbacterial prostatitis rat model. World J Mens Health. 2013; 31:150–156.

25. Zhou JF, Xiao WQ, Zheng YC, Dong J, Zhang SM. Increased oxidative stress and oxidative damage associated with chronic bacterial prostatitis. Asian J Androl. 2006; 8:317–323.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download