Abstract
Purpose
We compared a transperineal ligation model and a transperitoneal ligation model in male rats to determine which animal model of overactive bladder (OAB) was more useful based on cystometrography, estimations of oxidative stress, and measurements of pro-inflammatory cytokine levels.
Materials and Methods
Male rats were randomly divided into three groups (n=15 in each): the control group, the transperineal ligation group, and the transperitoneal ligation group. Four weeks after the ligation procedure, cystometrography was performed and oxidative stress, pro-inflammatory cytokine levels, and histologic changes were evaluated. Oxidative stress was assessed by measuring 8-hydroxy-20-deoxyguanosine and superoxide dismutase, and pro-inflammatory cytokine activity was investigated by measuring levels of interleukin (IL)-6, IL-8, and tumor necrosis factor-α.
Results
The transperineal model led to results similar to those observed for the transperitoneal model, namely (1) increased voiding frequency and reductions in the non-voiding contraction interval and the maximal vesical pressure, (2) increased levels of oxidative stress markers, (3) increased pro-inflammatory cytokine levels, and (4) fibrotic changes in the bladder tissue.
Benign prostatic hyperplasia (BPH) is one of the most common conditions in elderly men worldwide, and the resultant lower urinary tract symptoms can diminish the quality of life of men affected by BPH [12]. Autopsy studies have found BPH in approximately 40% of men aged 50 to 60 years, and 70% of men aged 60 to 70 years [3]. Overactive bladder (OAB), defined as a syndrome involving storage symptoms such as urgency (with or without urge incontinence), frequency, and nocturia, is also prevalent in men with BPH and poses a significant quality-of-life issue [456]. The pathophysiology of OAB can be explained through the effects of bladder outlet obstruction (BOO) due to BPH on the bladder urothelium, detrusor muscle, and neurologic function [7]. However, OAB symptoms often occur independently of BOO and persist in a considerable number of men, despite medical or surgical treatment [89].
Due to the legal and ethical problem involved in using human subjects for experiments, much of our understanding of human voiding function has stemmed from the use of animal models [10]. Currently, multiple animal models of OAB are used [11121314]. In addition, the effects of BOO in humans can be surgically induced in animal models. Such models have been established in a number of animals, including rabbits, pigs, and rats [151617]. Many studies of OAB have used partial BOO animal models. The most common method of inducing OAB in rats is narrowing the urethral diameter by silk ligation around the urethra through a transperitoneal approach [18]. However, previous studies have noted several disadvantages of the trans-peritoneal OAB model. The abdomen is opened through a midline incision and the tissue around the bladder is dissected, so injuries affecting bladder function can occur. Therefore, Zhang et al [19] introduced an improved model for OAB in female rats using a trans-perineal approach. This method involves making a suture around the perineal urethra to induce partial obstruction in female rats, and the researchers evaluated the effects of this model on bladder function. However, this technique has not been studied in male rats and, to the best of our knowledge, no studies have compared the trans-perineal ligation model and the transperitoneal ligation model in male rats.
Therefore, we sought to determine which model was more useful for establishing OAB and estimated the oxidative stress induced in male rats in the transperineal ligation model and the transperitoneal ligation model.
The treatment protocol was approved by the Institutional Animal Care and Use Committee of the School of Medicine, The Catholic University of Korea (CUMC-2015-0175-01). Sprague-Dawley male rats with a weight of 250 to 300 g were randomly divided into three groups of eight animals each, comprising the control group (n=15), the transperineal ligation group (n=15), and the transperitoneal ligation group (n=15).
Partial BOO was surgically induced via a transperineal ligation as previously described (Fig. 1) [19]. The experimental animals were anesthetized using an intramuscular injection of 40 mg/kg of ketamine and 20 mg/kg of xylazine with the rats in the supine position. The penile urethra was exposed and catheterized with a polyethylene tube through the urethral orifice (Fig. 1A). A needle with a 3-0 silk suture was used to make sutures penetrating around the perineal urethra and a partial obstruction was made by tying the urethra smoothly in the presence of the tube (Fig. 1B-1D). The tube was then removed (Fig. 1E), and the ligature around the urethra was removed after 4 weeks.
Partial BOO was surgically induced via a transperitoneal ligation as previously described [18]. The experimental animals were anesthetized using an intramuscular injection of 40 mg/kg of ketamine and 20 mg/kg of xylazine with the rats in the supine position. A 4-cm midline incision was made in the lower abdomen and the proximal urethra was dissected without damage. A polyethylene tube was placed beside the proximal urethra and a 3-0 silk ligation was performed smoothly in order to avoid inducing a total obstruction. The tube was removed and the incision was closed. The ligature around the urethra was removed after 4 weeks.
Cystometrography was performed in all groups. Rats were anesthetized using a subcutaneous injection of 1.2 mg/kg of urethane. A suprapubic midline laparotomy was made to expose the bladder, and a 25-G needle connected to polyethylene tubing was inserted into the bladder through the bladder dome. The tube was connected to a pressure transducer and a Harvard syringe pump via a three-way stopcock to record intravesical pressure and to infuse saline into the bladder. After emptying the bladder, cystometrography was performed using a saline infusion at a rate of 0.04 mL/min. The non-voiding contraction interval and contraction pressure were recorded using a polygraph (Grass 7D; Grass Institute Co., Quincy, MA, USA).
Oxidative stress in bladder tissue was assessed quantitatively by measuring levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) and superoxide dismutase (SOD). Total DNA was extracted from the bladder using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA). Levels of 8-OHdG were measured with a DNA oxidation kit (Highly Sensitive 8-OHdG Check ELISA; Japan Institute for the Control of Aging, Fukuroi, Japan). After the final color was developed with the addition of 3,3',5,5'-tetramethylbenzidine, absorbance was measured at 450 nm. Tissue sample concentration was calculated from a standard curve and was corrected for DNA concentration. SOD activity (CuZnSOD and MnSOD) in tissue was measured using a SOD Assay Kit-WST (Dojindo Laboratories, Kumamoto, Japan), monitoring the decrease in the rate of superoxide-mediated reduction of nitroblue tetrazolium at 450 nm using a spectrophotometer [20].
Blood obtained before sacrifice was centrifuged for 10 minutes (3,000 rpm, 4℃), and the supernatant was immediately transferred to a tube. Cytokine concentrations were measured every 5 minutes for 30 minutes, using a spectrophotometer at 450 nm with an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol [21].
Bladder tissue samples were fixed in 4% paraformaldehydefor one day at 4℃ and then embedded in paraffin. Subsequently, 7-µm thin slice sections were prepared for Masson's trichrome staining to observe the bladder muscle. After staining, the color distribution in the tissue was measuredusing Image Pro Plus version 5.0 (Mediacybernetics, Silver Spring, MD, USA). After the entire color distributionof the image was calculated, the muscle tissue was expressed using the color blue. The mean ratio of collagen and muscle fiber was calculated [22].
All data are presented as the mean±standard deviation. Statistical significance was evaluated using the Kruskal-Wallis test and the Mann-Whitney U-tests with the Bonferroni correction. Spearman's test was used for an analysis of correlations among cystometrography findings, levels of inflammation, measurements of oxidative stress, and cytokine levels. p<0.05 were considered to indicate statistical significance. All statistical analyses were conducted using the IBM SPSS software ver. 21.0 (IBM Co., Armonk, NY, USA).
The cystometrography results, oxidative stress levels, and pro-inflammatory cytokine levels were analyzed in the control group (n=15), the transperineal ligation group (n=15), and the transperitoneal ligation group (n=15). No significant differences in weight were found among the three groups. One rat died one week after the surgical procedure in the transperineal group (6.7%), 4 rats died within two weeks in the transperitoneal group (26.7%), and the ligature was removed in one rat in the transperineal group (6.7%), which was excluded from the study. Surgical time for the transperineal ligations was 32.0±7.1 seconds, in contrast to 217.0±48.4 seconds for the transperitoneal ligations (p<0.05).
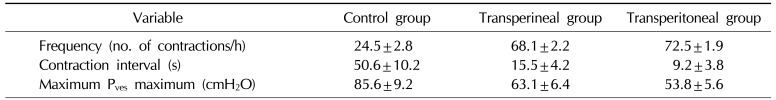
Cystometrography was performed in 39 male rats, equally divided among the control group, the transperineal group, and the transperitoneal group. Table 1 shows the results for the cystometrography parameters, including frequency (number of non-voiding contractions per hour), the non-voiding contraction interval, and maximal vesical pressure. Fig. 2 shows representative cystometrography tracings that are regular waveforms. Frequency was greater in both ligated groups than in the control group (p<0.05), and the non-voiding contraction interval and maximal vesical pressure were significantly smaller (p<0.05). The transperineal group exhibited smaller differences in comparison to the control group than the transperitoneal group (p<0.05).
The mean 8-OHdG and SOD levels in the bladder tissue are shown in Fig. 3. Higher 8-OHdG levels and lower SOD levels were found in both ligated groups than in the control group (p<0.05). Oxidative stress was higher in the ligated groups, and no significant differences were found between the transperineal group and the transperitoneal group.
Changes in pro-inflammatory cytokine levels in the three groups are shown in Fig. 4. The interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α levels were lower in the control group than in the ligated groups (p<0.05). No significant differences were found between the transperineal group and the transperitoneal group.
Fig. 5 shows the histologic characteristics of the three groups as visualized in an image analysis program. In the control group, the mucosa and smooth muscle appeared normal. However, the transperineal group showed inflammatory changes and fibrosis of the bladder tissue. Therefore, the ratio of smooth muscle to collagen was lower in the transperineal group than in the control group. Furthermore, the transperitoneal group exhibited more progressive fibrosis and lacked smooth muscle, indicating the occurrence of severe inflammation. Therefore, the ratio of bladder smooth muscle to collagen was reduced in both ligated groups, and no significant differences were noted between the transperineal group and the transperitoneal group.
The main findings of this study were as follows: (1) Cystometrography increased frequency and a decreased non-voidingcontraction interval and maximal vesical pressure in the ligated groups compared to the control group; (2) high oxidative stress in the both ligated group which increases in the oxidative stress markers 8-OHdG and decrease in the SOD levels. (3) higher levels of the pro-inflammatory cytokines IL-6, IL-8, and TNF-α were found in the ligated groups; and (4) histologic findings in the ligated groups indicated a decrease in the smooth muscle component and an increase in fibrotic tissue, meaning that the ratio of smooth muscle to collagen was lower than in the control group.
The characteristics of the transperineal model may be summarized as follows.
This model showed similar results to the transperitoneal model with regard to cystometrography, oxidative stress, cytokine levels, and histologic changes. Although the transperineal group exhibited smaller changes than the transperitoneal group, these changes were still statistically significant.
The procedure was simple and time-saving (the surgical time was 32±7.1 seconds versus 217.0±48.4 seconds, p< 0.05). Additionally, it is more reproducible than the transperitoneal procedure because it does not require a midline incision and wound closure. Therefore, the surgical procedure time was greatly reduced.
The method is more physiologic because it minimizes periurethral nerve and blood vessel injuries, which may result from midline incisions and cause bladder impairment. Blood supply and innervation are important for normal bladder function, meaning that such injuries can cause unnecessary and unpredictable variations in OAB models [19].
This new model was less invasive and had a higher survival rate than the transperitoneal procedure, with a survival rate of 93% versus 73%.
The transperineal model for OAB was introduced by Zhang et al [19] and applied to female rats. We therefore applied the same model to male rats. As described above, we obtained similar results to those obtained using a conventional transperitoneal model. Therefore, the transperineal model can be used as an alternative in further studies. An altered voiding pattern is essential for an OAB model. The transperineal model showed an altered voiding pattern in cystometrography and a progression of fibrosis in histology, as well as sufficient oxidative stress and an adequate inflammatory response. Obstructive bladder dysfunction generates reactive oxygen species (ROS) by cyclic ischemia/reperfusion injuries, and ROS-mediated oxidative reactions cause bladder wall denervation and instability [23]. Therefore, this model can be utilized in developing antioxidants for the treatment of bladder dysfunction secondary to BOO.
However, our study had several limitations. First, the OAB parameters changed less in the transperineal model than in the transperitoneal model. Therefore, this model was less effective in simulating OAB, although it nonetheless demonstrated statistically significant alterations. Second, erectile dysfunction cannot be evaluated using the transperineal ligation model, since cavernous injury is inevitable. Third, ligation is somewhat risky since ligatures can be removed by the movement of the rats. In our study, the ligature was removed in one case (6.7%). Fourth, the total number of rats was small, although the results were statistically significant. Therefore, additional studies are needed using larger numbers of rats to confirm our statistical findings. Fifth, additional studies are needed to assess blood vessel and nerve injuries in the transperineal model compared with the transperitoneal model and how they may affect the physiologic function of the bladder. Moreover, new procedures should be developed to avoid cavernous injuries, as well as mechanisms or devices to prevent ligature removal.
Our results showed that the transperineal procedure had similar results to the transperitoneal procedure in terms of cystometrography, oxidative stress, cytokine levels, and histologic changes. Moreover, this model was more convenient, more physiologic, and less invasive. Therefore, the transperineal procedure can be used as an alternative OAB model in male rats.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 Program (no. PJ011290012016), Rural Development Administration, Republic of Korea.
References
1. Sarma AV, Wei JT, Jacobson DJ, Dunn RL, Roberts RO, Girman CJ, et al. Comparison of lower urinary tract symptom severity and associated bother between communitydwelling black and white men: the olmsted county study of urinary symptoms and health status and the flint men's health study. Urology. 2003; 61:1086–1091. PMID: 12809866.

2. Verhamme KM, Dieleman JP, Bleumink GS, van der Lei J, Sturkenboom MC, Artibani W, et al. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care: the triumph project. Eur Urol. 2002; 42:323–328. PMID: 12361895.
3. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132:474–479. PMID: 6206240.

4. Peters TJ, Donovan JL, Kay HE, Abrams P, de la Rosette JJ, Porru D, et al. The international continence society "Benign Prostatic Hyperplasia" study: the botherosomeness of urinary symptoms. J Urol. 1997; 157:885–889. PMID: 9072592.
5. Eckhardt MD, van Venrooij GE, van Melick HH, Boon TA. Prevalence and bothersomeness of lower urinary tract symptoms in benign prostatic hyperplasia and their impact on well-being. J Urol. 2001; 166:563–568. PMID: 11458069.

6. Dmochowski RR, Staskin D. Overactive bladder in men: special considerations for evaluation and management. Urology. 2002; 60:56–62. PMID: 12493356.

7. Roosen A, Chapple CR, Dmochowski RR, Fowler CJ, Gratzke C, Roehrborn CG, et al. A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol. 2009; 56:810–819. PMID: 19683859.

8. de Nunzio C, Franco G, Rocchegiani A, Iori F, Leonardo C, Laurenti C. The evolution of detrusor overactivity after watchful waiting, medical therapy and surgery in patients with bladder outlet obstruction. J Urol. 2003; 169:535–539. PMID: 12544303.

9. Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014; 43:604–615. PMID: 25038833.
10. Gillespie JI. A developing view of the origins of urgency: the importance of animal models. BJU Int. 2005; 96(Suppl 1):22–28. PMID: 16086676.

11. Wróbel A, Łańcut M, Rechberger T. A new model of detrusor overactivity in conscious rats induced by retinyl acetate instillation. J Pharmacol Toxicol Methods. 2015; 74:7–16. PMID: 25957030.

12. Juszczak K, Ziomber A, Wyczolkowski M, Thor PJ. Urodynamic effects of the bladder C-fiber afferent activity modulation in chronic model of overactive bladder in rats. J Physiol Pharmacol. 2009; 60:85–91. PMID: 20065501.
13. Takeda H, Yamazaki Y, Igawa Y, Kaidoh K, Akahane S, Miyata H, et al. Effects of beta(3)-adrenoceptor stimulation on prostaglandin E(2)-induced bladder hyperactivity and on the cardiovascular system in conscious rats. Neurourol Urodyn. 2002; 21:558–565. PMID: 12382247.
14. Okutsu H, Matsumoto S, Ohtake A, Suzuki M, Sato S, Sasamata M, et al. Effect of tamsulosin on bladder blood flow and bladder function in a rat model of bladder over distention/emptying induced bladder overactivity. J Urol. 2011; 186:2470–2477. PMID: 22019173.

15. Drake M, Gillespie J, Hedlund P, Harvey I, Lagou M, Andersson KE. Muscarinic stimulation of the rat isolated whole bladder: pathophysiological models of detrusor overactivity. Auton Autacoid Pharmacol. 2006; 26:261–266. PMID: 16879491.

16. Kato K, Wein AJ, Kitada S, Haugaard N, Levin RM. The functional effect of mild outlet obstruction on the rabbit urinary bladder. J Urol. 1988; 140:880–884. PMID: 3418826.

17. Wolffenbuttel KP, Kok DJ, Minekus JP, van Koeveringe GA, van Mastrigt R, Nijman JM. Urodynamic follow-up of experimental urethral obstruction in individual guinea pigs. Neurourol Urodyn. 2001; 20:699–713. PMID: 11746551.

18. Mattiasson A, Uvelius B. Changes in contractile properties in hypertrophic rat urinary bladder. J Urol. 1982; 128:1340–1342. PMID: 7154206.

19. Zhang NZ, Ma L, Zhang JB, Chen J. Improved model for the establishment and evaluation of detrusor overactivity in female Wistar rats. Int Braz J Urol. 2014; 40:414–421. discussion 422. PMID: 25010309.

20. Bae WJ, Ha US, Kim KS, Kim SJ, Cho HJ, Hong SH, et al. Effects of KH-204 on the expression of heat shock protein 70 and germ cell apoptosis in infertility rat models. BMC Complement Altern Med. 2014; 14:367. PMID: 25269420.

21. Yoon BI, Bae WJ, Kim SJ, Kim HS, Ha US, Sohn DW, et al. The anti-inflammatory effects of a new herbal formula (WSY-1075) in a nonbacterial prostatitis rat model. World J Mens Health. 2013; 31:150–156. PMID: 24044110.

22. Bae WJ, Ha US, Choi JB, Kim KS, Kim SJ, Cho HJ, et al. Protective effects of KH-204 in the bladder of androgen-deprived rats. World J Mens Health. 2015; 33:73–80. PMID: 26331123.

23. Oka M, Fukui T, Ueda M, Tagaya M, Oyama T, Tanaka M. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol. 2009; 182:382–390. PMID: 19447421.

Fig. 1
Surgical procedure for the transperineal model. (A) Catheterization of a polyethylene tube through the urethral orifice. (B) A needle with a 3-0 silk suture was used to make a suture penetrating around the perineal urethra. (C) A knot was made smoothly in the presence of the tube. (D) Unnecessary thread was removed. (E) The polyethylene tube was removed.
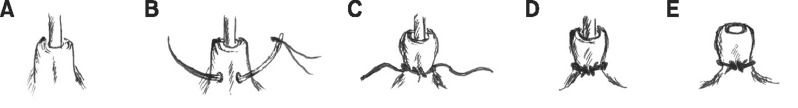
Fig. 2
Representative cystometrography tracings showing the changes in voiding function in the control group (n=15), the transperineal group (n=13), and the transperitoneal group (n=11).
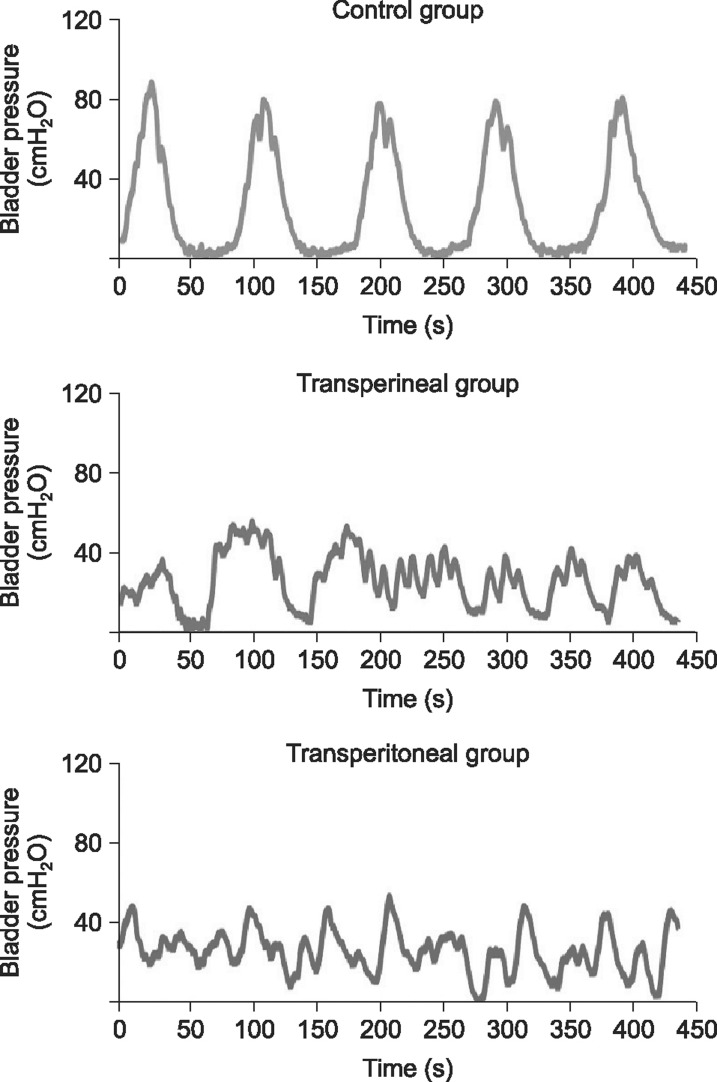
Fig. 3
Comparison of oxidative stress in bladder tissue from the control group (n=15), the transperineal group (n=13), and the transperitoneal group (n=11). 8-OHdG: 8-hydroxy-2-deoxyguanosine, SOD: superoxide dismutase. *p< 0.05.
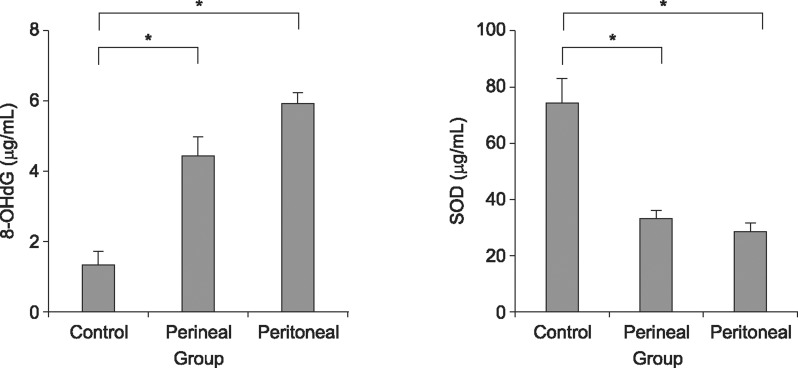
Fig. 4
Comparison of cytokine levels in bladder tissue from the control group (n=15), the transperineal group (n=13), and the transperitoneal group (n=11). IL: interleukin, TNF: tumor necrosis factor. *p<0.05.
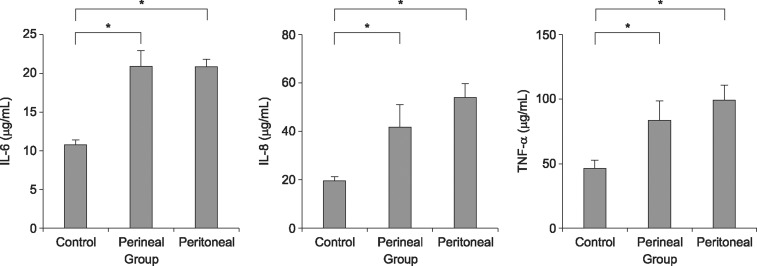
Fig. 5
Comparison of histologic findings of representative sections of bladder tissue stained with H&E (×200). (A) The control group (n= 15). (B) The transperineal group (n=13). (C) The transperitoneal group (n=11). (D) Ratio of smooth muscle to collagen in the bladder. *p< 0.05.
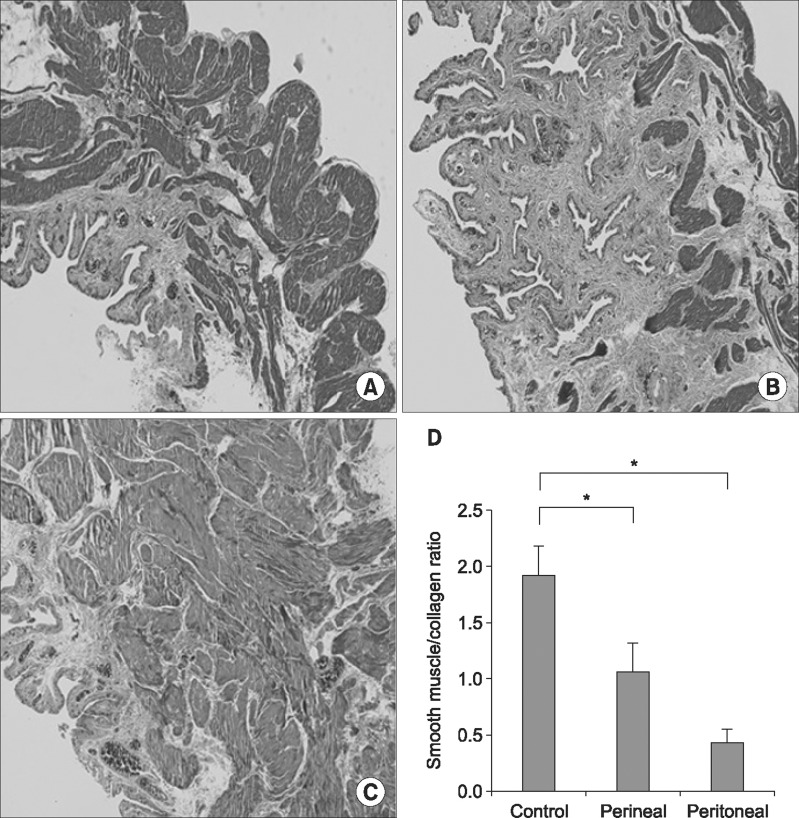
Table 1
Results of the cystometrographic analysis of the control group (n=15), the transperineal group (n=13), and the transperitoneal group (n=11)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download