Abstract
Purpose
We investigated the protective effects of the herbal formulation KH-204 in the bladder of androgen-deprived rats.
Materials and Methods
Male rats aged eight weeks were randomly divided into four groups, containing eight rats each: sham operation only (normal control group), androgen-deprived only (androgen-deprived control group), and androgen-deprived followed by treatment with 200 mg/kg or 400 mg/kg of KH-204. After 0.5 mg/kg of leuprorelin was subcutaneously injected in the androgen-deprived groups, the oral administration of either distilled water in the two control groups or KH-204 in the treatment group was continued for four weeks. Serum testosterone levels, RhoGEF levels, nitric oxide (NO)-cyclic guanosine monophosphate (cGMP)-related parameters, oxidative stress, and histologic changes were evaluated after treatment.
All physiological functions progressively decline with age, as exemplified by muscle mass loss, decreased bone mineral density, depression, and sexual dysfunction. Late-onset hypogonadism (LOH) is a clinical and biochemical syndrome correlated with aging that is characterized by symptoms reflecting deficient serum testosterone levels [1]. Erectile dysfunction (ED), weakened rigidity of morning erections, and decreased sexual desire are the main symptoms of LOH and play a crucial role in its diagnosis [2]. Most older men have reduced levels of serum testosterone, which is a primary cause of the symptoms of LOH [3]. Several studies have reported that serum testosterone levels begin to decline steadily at 30 to 40 years of age, at a rate of about 1% every year [45]; nonetheless, 20% of men over 60 years of age and 50% of men over 80 years of age have lower serum testosterone levels than would be expected [6].
It is also common for aging men to experience lower urinary tract symptoms (LUTS). A progressive enlargement of the prostate, known as benign prostatic hyperplasia (BPH), is associated with the decline in testosterone that occurs beginning in middle age [7]. Recent studies have suggested that LUTS are significantly correlated with decreased testosterone levels as well as with BPH. These studies have suggested that testosterone levels likely affect the autonomic nervous system and the activities of nitric oxide synthase (NOS) and phosphodiesterase-5, and that these mechanisms may affect urinary function via the androgen receptors localized in the lower urinary tract [891011].
In light of these findings, androgen replacement therapy (ART) appears to be a useful treatment for LOH. However, treatment with artificial testosterone can also lead to many side effects that can worsen LUTS by promoting BPH or prostate cancer [12]. Therefore, in Korea, various nutritional supplements and phytotherapies are often used to treat LOH in order to avoid the adverse effects of ART.
Schisandra chinensis Baillon, Rubus coreanus Miquel, Cuscuta chinensis Lam, and Lycium chinense Mill are herbs that are widely used in Korean traditional medicine, and they are combined in the herbal formulation known as ojayeonjonghwan, which is used to treat LOH symptoms, including ED [13]. A previous animal study demonstrated that this formulation improved ED via the restoration or activation of the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway, with the synergistic effect of activating NOS. Recently, we performed a study investigating a modified form of ojayeonjonghwan, KH-204, evaluating its protective effect on heat stress-induced oxidative damage in rat testes [14].
We hypothesized that positive effects of KH-204 on the urinary bladder would be attributable to its antioxidant effects or to an elevation in NO-cGMP activity. We investigated the protective effects of KH-204 on the bladder of androgen-deprived rats.
The major ingredients of KH-204 are seeds obtained from five plants: Cornus officinalis (32%), Lycium chinense (32%), Rubus coreanus (16%), Cuscuta chinensis (16%), and Schisandra chinensis (4%). KH-204 was manufactured as described previously [14]. A venture company developing oriental herbal medicines, the Korea Biomedical Science Institute (Andong, Korea), developed this product as a health supplement.
The protocol was approved by the Institutional Animal Care and Use Committee in School of Medicine, The Catholic University of Korea (CUMC-2015-0035-02). Sprague-Dawley male rats aged eight weeks were randomly divided into four groups of eight animals each, which underwent 1) a sham operation only (normal control group), 2) androgen deprivation only (androgen-deprived control group), 3) androgen deprivation followed by treatment with 200 mg/kg of KH-204, or 4) androgen deprivation followed by treatment with 400 mg/kg of KH-204. We administered either distilled water (normal control group) or 0.5 mg/kg of leuprorelin subcutaneously one time to the backs of the rats. In each group, the once-daily oral administration of either distilled water (normal control group, androgen-deprived control group) or KH-204 (treatment groups) was continued for four weeks. KH-204 was dissolved in distilled water and administered orally through an 8-F red Rob-Nel catheter once a day, as described previously [14]. The dosage of leuprorelin was selected by referring to the results of a previous experiment [15]. Even at the maximum dose (2.8 mg/kg) tested in that study, leuprorelin-related effects were less marked than castration-related effects. We chose our dosage to simulate partial androgen deficiency in aging males. The animals in all groups were sacrificed four weeks after treatment, and blood samples were collected. Their bladders were removed and weighed.
We extracted total RNA (2 µg per reaction) from rat bladder tissue using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and performed cDNA synthesis using the SuperScript III First-Strand kit (Invitrogen) according to the manufacturer's instructions. PDZ-RhoGEF, leukemia-associated RhoGEF, and p115RhoGEF were used as published primers [16], and a β-actin primer was designed to bind an exon-exon base. In research on diabetes, β-actin has been used as a housekeeping gene because its expression is unaffected by glucose. The polymerase chain reaction (PCR) amplification of cDNA was performed in a real-time PCR machine (Perkin-Elmer Applied Biosystems, Wellesley, MA, USA) with the SYBRGreen PCR master mix (Invitrogen) as indicated: two minutes at 50℃ for dUTP activation, 10 minutes at 95℃ for the initial denaturation of cDNA, followed by 40 cycles, each consisting of 15 seconds of denaturation at 95℃ and 60 seconds at 60℃, for primer annealing and chain extension.
The primer pairs were the following: NaPi 2a, forward, 5'-GCCACTTCTTCTTCAACATC-3'; reverse, 5'-CACAC GAGGAGGTAGAGG-3'; cyclo A forward, 5'-CAAAGTT CCAAAGACAGCAGAAAA-3'; reverse, 5'-CCACCCTGG CACATGAAT-3'.
Frozen bladder tissue was ground to a fine powder with a liquid nitrogen-cooled mortar and pestle. The bladder total protein was extracted using a cell lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride). Protein extracts were quantified with the BCA Protein Assay reagent (Thermo Scientific, Rockford, IL, USA). Quantitative proteins (30 µg) were boiled in the loading buffer (62.6 mM tris-HCl pH 6.8, 2% sodium dodecyl sulfate [SDS], 0.01% bromophenol blue, 10% glycerol, and 100 mM DTT). Proteins were loaded into each lane and resolved by 4% to 12% SDS-polyacrylamide gel electrophoresis. Proteins were transferred onto Hybond-ECL nitrocellulose membranes (Amersham Biosciences, Freiburg, Germany), and equal protein loading was verified by Ponceau-S staining (Sigma Aldrich Co., St. Louis, MO, USA). The membranes were blocked by treatment with 5% non-fat milk in tris-buffered saline containing 0.1% Tween 20, and membranes membranes were probed with endothelial NOS (1:1,000, BD Pharmingen, San Diego, CA, USA), neuronal NOS (1:1,000, BD Pharmingen), and anti-β-Actin antibody (1:10,000; Perkin-Elmer Applied Biosystems). The densitometric analysis of band intensity used the Luminescent Image Analysis System (LAS-3000; Fujifilm, Tokyo, Japan).
The measurement of serum testosterone concentrations was performed using an enzyme-linked immunosorbent assay (ELISA) testosterone detection kit (BioVendor-Laboratory Medicine Inc., Brno, Czech Republic). Similarly, the measurement of cGMP concentrations in bladder tissue was performed using an ELISA cGMP detection kit (R&D Systems, Minneapolis, MN, USA).
Superoxide dismutase (SOD) activity (CuZnSOD and MnSOD) was measured by a SOD Assay Kit-WST (Dojindo Laboratories, Kumamoto, Japan) that monitored the decrease in the rate of superoxide-mediated reduction of nitroblue tetrazolium at 450 nm using a spectrophotometer.
Bladder tissue samples were fixed in 4% paraformaldehyde for one day at 4℃ and then embedded in paraffin. Subsequently, 7-µm thin slice sections were prepared for Masson's trichrome staining to observe the bladder muscle. After staining, the color distribution in the tissue was measured using Image Pro Plus, version 5.0 (Mediacybernetics, Silver Spring, MD, USA). After the entire color distribution of the image was calculated, the muscle tissue was expressed as the color blue. The mean ratio of collagen and muscle fiber was calculated.
Statistical analyses were performed and graphs were created using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean±standard deviation. Statistical significance was determined via analysis of variation, with group comparisons made by Scheffe's test. p values <0.05 were considered to indicate statistical significance.
No significant differences were observed in body weight after four weeks. Similarly, the mean bladder weight after treatment was 0.135±0.001 g in the normal control group, 0.141±0.012 g in the androgen-deprived control group, 0.137±0.005 g in the group treated with 200 mg/kg of KH-204, and 0.139±0.010 g in the group treated with 400 mg/kg of KH-204. These differences were not significant.
The serum testosterone levels after treatment in the normal control group, androgen-deprived control group, the group treated with 200 mg/kg of KH-204, and the group treated with 400 mg/kg of KH-204 were 2.484±0.413 ng/mL, 1.287±0.342 ng/mL, 1.321±0.524 ng/mL, and 1.719±0.15 ng/mL, respectively (Fig. 1). These values indicate that serum testosterone levels decreased after androgen deprivation and were then increased by treatment with 400 mg/kg of KH-204 (p<0.05).
The expression of the three RhoGEFs in the androgen-deprived group was significantly higher than in the normal control group (Fig. 2). In addition, their expression in the treatment group was significantly decreased compared to the androgen-deprived group (p<0.05).
The expression of endothelial NOS, neuronal NOS, and cGMP in the androgen-deprived group was significantly decreased compared to the normal control group (Fig. 3). Dose-dependent increases in NOS expression were observed in the treatment group (p<0.05). The expression of cGMP in the treatment group was also increased, but not significantly.
The mean SOD expression in the bladder is shown in Fig. 4. Expression in the androgen-deprived control group decreased significantly, and dose-dependent increases in expression were observed in the treatment groups. Oxidative stress was found to be significantly lower in the group treated with 400 mg/kg of KH-204 than in the androgen-deprived control group (p<0.05).
An increase in the mean thickness of the muscularis layer was not observed in any group. We observed increased bladder fibrosis in the androgen-deprived control group compared to the normal control group (Fig. 5), and this effect was attenuated by KH-204 treatment. In addition, an increased ratio of collagen to smooth muscle in the androgen-deprived control group compared to the normal control group was found in image analysis, indicating increased bladder fibrosis. However, after KH-204 treatment, this increased ratio was attenuated.
Although the mechanisms of aging remain unclear, it is generally accepted that oxidative stress plays an important role in the aging process [16]. The free radical theory of aging proposes that oxygen free radicals, which are byproducts of aerobic metabolism, cause cumulative oxidative stress damage, which ultimately results in aging [17]. We demonstrated that KH-204 treatment can decrease oxidative stress in heat stress-induced rats and hypothesized that it could suppress the production of reactive oxygen species [14]. In the present study, the level of SOD increased significantly in the group treated with 400 mg/kg of KH-204.
Most studies have found that bladder weight increases in aging rats [1819]. The bladder tissue seems to have a thickened muscularis layer, similar to what is observed in BPH. In contrast to the rats described in other studies, we did not find vesicle hypertrophy in this study. However, we did identify structural changes from androgen deprivation leading to bladder fibrosis, and treatment with KH-204 alleviated these changes.
Aging men experience LUTS, which can be multifactorial. The frequency of moderate to severe LUTS in men has been shown to increase with age in population studies, and some researchers are currently investigating the relationship between metabolic syndrome and overactivity of the autonomic nervous system [2021]. In some experimental animal studies, testosterone concentration seemed to play a major role in pelvic autonomic neurons, and castration was found to impair alpha 1-adrenergic and muscarinic receptors in the urinary bladder [822].
The activated Rho-kinase system also increases smooth muscle contraction, leading to decreased erectile function and changes in bladder muscle tone [23]. Testosterone has been shown to affect Rho-kinase activity, and its activity in the urinary tract could be partially dependent on testosterone [9]. Experimentally, Rho-kinase has been found to play an important role in the control of smooth muscle contraction of the urinary bladder and is associated with calcium sensitivity of the contractile system in the detrusor muscle [10]. Increased Rho-kinase activity occurs simultaneously with the progress of LUTS in aging men with BPH, and inhibition of Rho-kinase in rat models has been examined to determine if it decreases prostatic smooth muscle contraction and cell proliferation [24]. The results of the present study confirm those of previous studies: expression of Rho-kinase was increased in the androgen-deprived control group compared to the normal control group, and it significantly decreased in the group treated with 400 mg/kg of KH-204.
In addition, NO is a major factor modulating the effects of testosterone levels on LUTS. A previous study demonstrated that NO is an important nerve-induced mediator of erection and the micturition reflex, but NO may also be involved in several other functions in the human urogenital tract [25]. NO mediates dilatation of the urinary tract, and NOS expression could be associated with smooth muscle tone in relation to LUTS [10]. Park et al [13] demonstrated that KH-204 treatment increased penile expression levels of NOS and cGMP concentrations in aged rats. We also found that KH-204 treatment activated NO expression, leading to an increase in the synthesis of cGMP, which improved the muscle/collagen ratio as assessed using Masson's trichrome stain. Furthermore, ART activates endothelial NOS and therefore increases the NO concentration in the pelvic vessels, resulting in the alleviation of pelvic ischemia [26]. However, the relationship between ART and the risk of progression of BPH is controversial. A previous study found that ART resulted in an increase in prostate volume after eight months of treatment, but also found that it did not affect uroflowmetry data or LUTS [27]. A long-term clinical study using oral ART found a negligible decrease in urine flow but no increase in prostate volume [28]. As a result, few long-term observational studies of ART have included patients with severe BPH or LUTS. Our study found that testosterone levels were restored by KH-204 treatment after androgen deprivation, which suggests that KH-204 may be a useful treatment for managing LOH. Our findings suggest that the efficacy of KH-204 may be attributable to its antioxidant effects or to an elevation in NO-cGMP activity.
We note that our study has some limitations. We did not gather functional data for the bladder (cystometry) for each treatment group. Such data could have provided better insight into the effects of KH-204 in androgen-deprived rats. Moreover, we found no clear link between the antioxidant effects of KH-204 and other results, such as the activation of the NO-cGMP pathways or the decrease of Rho-kinase expression. Future work should investigate the functional outcomes and detailed mechanisms of the effect of KH-204 on LOH bladders.
Our results indicate that the expression of Rho kinase is increased and the expression of cGMP is decreased in androgen-deprived rat bladder. However, these impairments improved after treatment with KH-204, which was accompanied by an alteration in oxidative stress patterns. This herbal formulation reduces oxidative stress and was able to prevent deleterious molecular changes of the bladder in an LOH rat model.
Figures and Tables
 | Fig. 1Serum testosterone levels increased by KH-204 treatment. Control: sham operation only, Androgen-dep.: androgen-deprived control group, 200 mg/kg: androgen deprivation followed by treatment with 200 mg/kg of KH-204, 400 mg/kg: androgen-deprivation followed by treatment with 400 mg/kg of KH-204. *p<0.05. |
 | Fig. 2Comparison of the mRNA expression of RhoGEFs in the bladder among the four groups. LARG: leukemia-associated RhoGEF, Control: sham operation only, Androgen dep.: androgen-deprived control group, 200 mg/kg: androgen deprivation followed by treatment with 200 mg/kg of KH-204, 400 mg/kg: androgen-deprivation followed by treatment with 400 mg/kg of KH-204. *p<0.05. |
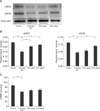 | Fig. 3Comparison of the expression levels of eNOS, nNOS and cyclic guanosine monophosphate (cGMP). (A, B) Densitometric analysis of eNOS and nNOS relative to beta-actin and (C) cGMP concentration in bladder tissue. Control: sham operation only, Androgen dep.: androgen-deprived control group, 200 mg/kg: androgen deprivation followed by treatment with 200 mg/kg of KH-204, 400 mg/kg: androgen-deprivation followed by treatment with 400 mg/kg of KH-204, eNOS: endothelial nitric oxide synthase, nNOS: neuronal nitric oxide synthase. *p<0.05. |
 | Fig. 4Comparison of the expression levels of oxdidative stress as reflected by total superoxide dismutase (SOD) expression in bladder tissue. Control: sham operation only, Androgen dep.: androgen-deprived control group, 200 mg/kg: androgen deprivation followed by treatment with 200 mg/kg of KH-204, 400 mg/kg: androgen-deprivation followed by treatment with 400 mg/kg of KH-204. *p<0.05. |
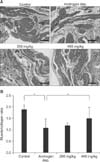 | Fig. 5Comparison of histologic findings among the four groups. (A) Masson's trichrome staining. The scale bars shown in each figure represent 100 µm. (B) Bladder smooth muscle/collagen ratio. Control: sham operation only, Androgen dep.: androgen androgen-deprived control group, 200 mg/kg: androgen deprivation followed by treatment with 200 mg/kg of KH-204, 400 mg/kg: androgen-deprivation followed by treatment with 400 mg/kg of KH-204. *p<0.05. |
ACKNOWLEDGEMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 Program (no. PJ011290012015), Rural Development Administration, Republic of Korea.
References
1. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. International Society of Andrology. International Society for the Study of Aging Male. European Association of Urology. European Academy of Andrology. American Society of Andrology. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009; 55:121–130.

2. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010; 363:123–135.

3. Wu CY, Yu TJ, Chen MJ. Age related testosterone level changes and male andropause syndrome. Chang Gung Med J. 2000; 23:348–353.
4. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002; 87:589–598.

5. Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008; 93:2737–2745.

6. Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002; 57:M76–M99.

7. Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol. 2011; 52:657–663.

8. Madeiro A, Girão M, Sartori M, Acquaroli R, Baracat E, Rodrigues De Lima G. Effects of the association of androgen/estrogen on the bladder and urethra of castrated rats. Clin Exp Obstet Gynecol. 2002; 29:117–120.
9. Pradidarcheep W. Lower urinary tract symptoms and its potential relation with late-onset hypogonadism. Aging Male. 2008; 11:51–55.

10. McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int. 2006; 97:Suppl 2. 23–28. discussion 44-5

11. Gomelsky A, Dmochowski RR. Urodynamic effects of once-daily tadalafil in men with LUTS secondary to clinical BPH. Curr Urol Rep. 2010; 11:254–260.

12. Barqawi A, Crawford ED. Testosterone replacement therapy and the risk of prostate cancer. Is there a link? Int J Impot Res. 2006; 18:323–328.

13. Park CS, Ryu SD, Hwang SY. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of aged and diabetic rats. J Ethnopharmacol. 2004; 94:85–92.

14. Bae WJ, Ha US, Kim KS, Kim SJ, Cho HJ, Hong SH, et al. Effects of KH-204 on the expression of heat shock protein 70 and germ cell apoptosis in infertility rat models. BMC Complement Altern Med. 2014; 14:367.

15. Gotanda K, Shinbo A, Okada M, Nakano Y, Kobayashi H, Sasaki T, et al. Effects of combination therapy with a luteinizing hormone-releasing hormone agonist and chlormadinone acetate on rat prostate weight and plasma testosterone levels. Prostate Cancer Prostatic Dis. 2003; 6:66–72.

16. Troen BR. The biology of aging. Mt Sinai J Med. 2003; 70:3–22.
18. Chun AL, Wallace LJ, Gerald MC, Levin RM, Wein AJ. Effect of age on in vivo urinary bladder function in the rat. J Urol. 1988; 139:625–627.

19. Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, et al. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol. 2000; 278:R964–R972.
20. Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol. 2008; 26:359–364.

21. Rohrmann S, Nelson WG, Rifai N, Kanarek N, Basaria S, Tsilidis KK, et al. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III). Urology. 2007; 69:708–713.

22. Takyu S. Effects of testosterone on the autonomic receptor-mediated function in lower urinary tract from male rabbits. Nihon Hinyokika Gakkai Zasshi. 1993; 84:330–338.

23. Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006; 147:Suppl 2. S41–S55.

24. Rees RW, Foxwell NA, Ralph DJ, Kell PD, Moncada S, Cellek S. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003; 170:2517–2522.

25. Ehrén I, Adolfsson J, Wiklund NP. Nitric oxide synthase activity in the human urogenital tract. Urol Res. 1994; 22:287–290.
26. Yassin DJ, El Douaihy Y, Yassin AA, Kashanian J, Shabsigh R, Hammerer PG. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014; 32:1049–1054.

27. Holmäng S, Mårin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate. 1993; 23:99–106.

28. Gooren LJ. A ten-year safety study of the oral androgen testosterone undecanoate. J Androl. 1994; 15:212–215.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download