Abstract
Glans penis augmentation (GPA) has received little attention from experts despite the existence of a subset of patients who may be dissatisfied with a small glans or poor tumescence of the glans during erection. Recently, GPA using an injectable filler or implantation of a graft or filler has been developed. Despite a demanding injection technique and inevitable uneven undulation of the glandular surface, GPA using injectable hyaluronic acid (HA) gel is a novel and useful therapy and an effective and safe procedure for soft tissue enhancement. For long-term presence of implants, timed supplementation can be used similar to that for fascial plasty. In complications such as mucosal necrosis of the glans penis, most cases occur from the use of non-HA gel or an unpurified form and misunderstanding of the management protocol for immediate side effects. Currently, GPA using injectable HA gel is not recommended in the International Society for Sexual Medicine guideline due to possible sensory loss. In a 5-year long-term follow-up of GPA by subcutaneous injection of HA gel, the residual volume of implants decreased by 15% of the maximal glandular circumference, but was still effective for alleviating the hypersensitivity of the glans penis in premature ejaculation patients. For efficacy in premature ejaculation, selection of appropriate candidates is the most important factor for success. GPA does not harm erectile function and is less invasive and irreversible compared to dorsal neurectomy. To refine the procedure, more interest and well-designed studies are required for the establishment of the procedure.
Although augmentation phalloplasty is not an established procedure, the demand for penile augmentation continues to increase as the media promotes a standard for normalcy among males and advertisements create interest in corrective surgery. The current main augmentation phalloplasty techniques are girth enhancement and penile lengthening procedures. There is no argument against glans penis augmentation (GPA) for hypospadias repair and penile reconstruction for congenital micropenis or secondary micropenis (iatrogenic, infection, or trauma). The current penile girth enhancement (PGE) technique may produce an iatrogenic penile deformity of a thick penile body with a relatively small glans penis. In addition to some patients' complaints of poor glans tumescence during normal erection [1], a small glans and lack of tumescence are evident after penile prosthesis implantation [23].
However, GPA remains controversial for subjective cases of small glans penis arising from a sense of sexual inferiority, small glans during erection, or premature ejaculation. There is no established GPA procedure for the improvement of sexual function. Moreover, clinically, most patients who seek penile enlargement request both penile elongation and girth enhancement with additional GPA, if possible. Therefore, many doctors and patients are showing an interest in GPA, but there is no safe and effective standard method for GPA. The reasons for the absence of a GPA standard are poor understanding of glans penis anatomy and consequent misconceptions of structural infeasibility, lack of an adequate filler material or implant, and lack of technical expertise. To develop a GPA method, the technical feasibility of subcutaneous injection of filler into the glans penis, presence of histological potential space in the glans penis, and understanding of the implications of the long-term presence of implants should be demonstrated [4]. The available modalities for glans augmentation have been confined to injection with fillers [567] or graft or filler implantation [8]. All of these will be introduced below.
Besides a sense of sexual inferiority, why do so many people want to have larger glans penis? What is the role of the glans penis? There is no description of the function of the glans penis in textbooks on andrology, urology, or even in anatomy and physiology. The penis is a social symbol of the male and is an apparatus for both voiding and sexual intercourse. As an apparatus for voiding, the essential role of the glans penis can be easily understood based on the purpose of hypospadias repair. The glans penis maintains a straight urinary stream and protects the distal urethra. As an apparatus for sexual intercourse, the glans penis is a sensory organ for sexual stimuli. Glans erection elongates the underlying elastic rete ridge, which exposes more underlying sensory receptors. As an apparatus for intercourse, the glans penis has a unique function based on its conical shape. The conical glans penis is the appropriate shape for vaginal dilatation and for easy intromission of the penis into the vagina. If the glans size is too small compared to a thicker shaft, intromission is not as easy or effective. The conical shape of the glans penis aids in aiming and is effective for transfer of axial cavernosal pressure to the distal tip of the penis. During erection, the cushion effects of the glans penis prevent both vaginal trauma and injury of the distal penis. For these reasons, the penis cannot function without the glans. Patients typically desire that the penis appear aesthetically normal, appropriately sized relative to the penile body, and as tumescent as rest of the penis during erection.
In 2003, Moon et al [4] first reported a novel GPA method using injectable hyaluronic acid (HA) gel following the same principles as wrinkle correction and reported three consecutive human studies of GPA for volume effects, premature ejaculation, and 5-year long-term follow-up results in 2008 [589]. After the development of GPA using injectable HA gel, various kinds of fillers have been used. In 2003, Perovic et al [6] reported results of GPA by submucosal injection of hydrogel in 9 patients with glans deformity. Shaeer [7] also used polyacrylamide gel injection for enhancing the glans size in 2 patients following implantation of a penile prosthesis with satisfactory results, although short lived, and thus requiring reinjection. Abdallah et al [10] reported modified GPA using injectable HA gel for premature ejaculation patients using a multiple puncture technique for simple injection of a large volume of filler in 2012.
Injectable soft-tissue substitutes provide an affordable, nonsurgical alternative for soft-tissue augmentation and correcting contour defects. The ideal filling substance for soft-tissue augmentation should be biocompatible, nonantigenic, nonpyrogenic, noninflammatory, nontoxic, easy to use, stable after injection, nonmigratory, long-lasting but resorbable, natural looking, and not too expensive [1112]. Since the late 1990s, HA has been shown to possess many properties that suggest its value in several medical applications, particularly in ophthalmology, orthopedics and recently, in soft-tissue augmentation in aesthetic surgery, with proven efficacy and safety [131415]. A ubiquitous component of all mammalian connective tissue, HA (also called hyaluronan), is a naturally occurring polysaccharide, with the same chemical and molecular composition in all species; and occurring in the intercellular matrix of dermal layers of the skin of all species. Therefore, HA sourced from animals can be used in humans, making it highly biocompatible without creating foreign body reactions [161718]. The HA matrix has an enormous ability to bind water (water content greater than 99%) and form hydrated polymers of high viscosity [19]. It resides in the extracellular space and functions as a space-filling, structure-stabilizing, and cell-protective molecule with uniquely malleable physical properties and superb biocompatibility. With these unique physical properties, HA has proved to be an ideal material for soft-tissue augmentation [20]. The amount of HA in the skin decreases with age, and loss of this substance results in reduced dermal hydration and increased folding [21]. Current uses of HA include supplementation of joints, wound healing, corrective surgery of facial deformity, and in the Deflux system of the urological field [22]. Restylane and Perlane (Q-Med, Uppsala, Sweden) are injectable HA gels and have the same composition of 20 mg/mL of stabilized HA gel. The difference between the products is the size of the gel particles. HA is a glycosaminoglycan biopolymer composed of alternating residues of the monosaccharides D-glucuronic acid and N-acetyl-D glucosamine linked in repeating units. The molecular weight of HA in its pure form can be determined. However, HA in its pure form is not stabilized. Injectable HA gel is an HA product chemically modified to increase its longevity in the tissue and to form a gel. It is not relevant to talk about molecular weight, as it cannot be determined for the stabilized gel.
Although HA has already been used in its native form as an implant for more than 30 years and in millions of individuals without causing adverse reactions in various areas of medicine, no reports of penile augmentation have been published. For the development of GPA using injectable HA gel, the feasibility of subcutaneous injection into the glans penis, the presence of potential space in the glans penis, and the long-term presence of implants with sustained volume effects should be demonstrated. The approximate number of gel particles is 100,000/mL in Restylane and 1,000/mL in Perlane, respectively. For this reason, Q-Med recommends a 30 G needle to inject Restylane into the mid-to upper part of the dermis and a 27 G needle to inject Perlane into the deep layer of the dermis.
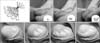
Based on the same principles of wrinkle correction, Moon et al [4] demonstrated the feasibility of subcutaneous injection of Restylane into the small glans penis of New Zealand White rabbits via 30 G needle and Perlane into the glans penis of Beagle dogs with a 27 G needle (Fig. 1). They also demonstrated the presence of potential space in the lamina propria and long-term presence without volume loss for 6 months in all animals (Fig. 1).
For human glans penis injection, local anesthesia 30 minutes after topical application of anesthetic cream Emla (lidocaine 25 mg, prilocaine 25 mg; AstraZeneca, London, UK) is tolerable for most patients, but a few experience penile pain and require local injection of anesthetic. To develop a simple and effective injection technique, the linear threading technique was introduced, but it required too many punctures, which can cause mucosal tearing, bleeding, and leakage through the needle site (Fig. 2). Thereafter, HA gel was injected by the fan technique (Fig. 3). Fewer needle punctures are required with this method. In both techniques, the injection needle was indwelled subcutaneously at one-third of the distance proximally from the tip of the glans to the coronal sulcus. In humans, it is not very difficult to inject HA into the dermis of the glans penis because the human glans is elastic and most surgeons are already familiar with this technique, which is frequently used to create a subcutaneous bulla for the skin test of hypersensitivity and to simplify dissection of subcutaneous tissues. To avoid over-injection, the appropriate volume of Perlane (HA gel; Q-Med) is 2 mL. Kim et al [5] injected supplemental Restylane (HA gel; Q-Med) via 30 G needle to correct the uneven undulation of the glandular surface. However, distribution of the gel through the whole glans penis is not particularly easy for the beginner with this injection technique. Unlike facial skin, the glans has multiple tiny folds originating from the underlying rete ridge and the augmented surface is inevitably uneven and looks unnatural. But the tiny folds and inevitable minor surface undulation disappear during erection.
Although not noted by Kim et al [5], it is very difficult to inject the ventral side close to the frenulum and the whole marginal area along the coronal sulcus. In 2012, Abdallah et al [10] developed the multiple puncture technique and compared it with the fan technique in their pilot study. They used multiple points of entry starting from the proximal one-third of the glans along the coronal sulcus together with the frenulum and injected only 0.25 mL at each point. They reported that their injection technique has an advantage over the fan technique in that it allows more uniform distribution of the injected material with less pain because the size of the bullae created is smaller than those created using the fan technique. To avoid too great a volume of injection initially and consequent discoloration or pressure necrosis, an initial injection of 2 mL of injectable HA gel (Perlane; Q-Med) via a 27 G needle and supplemental injection of Restylane via 30 G needle at 2 weeks after the initial injection was recommended by Kim et al [5].
To estimate the volume effects of GPA by injectable HA gel or other kinds of fillers, the long-term residual volume should be assessed. There is no established objective method to estimate the residual volume of implants or the long-term results of cosmetic surface augmentation. Even the most sensitive imaging study cannot measure the remnant volume accurately because of the relatively small injection volume, uneven distribution, and changes in the nature of implants through long-term tissue interaction. In light of these limitations, Kim et al [5] estimated the changes in glandular diameter, patient's subjective visual estimation of glandular size, and patient's satisfaction with efficacy and early and late complications. Change in glandular diameter was measured by tapeline to identify the net increase in the maximal glandular circumference after augmentation of the glans penis. The patient's subjective visual estimation of glandular size was solicited to assess the residual volume of HA gel. The patients responded on the basis of a visual analogue scale from Grade (Gr) 0 to Gr 4: Gr 0, no residual volume; Gr 1, less than 25% of initial volume; Gr 2, less than 50%; Gr 3, less than 75%; Gr 4, more than 75% or nearly the same as the initial volume. Patient satisfaction was also evaluated from Gr 0 to Gr 4, as follows: Gr 0, very dissatisfied; Gr 1, moderately dissatisfied; Gr 2, neutral; Gr 3, moderately satisfied; Gr 4, very satisfied. Any adverse reactions were also evaluated. In 100 patients with subjective small glans penis, the maximal glandular circumference was significantly increased compared to the baseline circumference, and the net increase in the maximal glandular circumference was 14.9±30.80 mm at 1 year after injection. In the patients' visual estimation, 38% and 57% of the patients estimated the residual volume to be more than 50% and 75%, respectively, of the initial volume, and 77% of the patients were satisfied with the outcome at one year after the procedure. In 87 patients with a small glans after PGE with a dermofat graft, the net increase in the maximal glandular circumference was 14.78±0.89 mm. In the visual estimation, 29.9% and 70.1% of the patients estimated the residual volume to be more than 50% and 75%, respectively, of the initial volume and 69% of the patients were satisfied. There was no abnormal reaction in the sensation, texture, or color of the area. In most cases, initial discoloration by glandular swelling recovered to normal within 2 weeks. Postoperative consistency of the glans penis was natural without deformity and maintained through one year. There were no signs of inflammation and no serious adverse reactions in any of the cases.
To evaluate the long-term residual volume of implants and their efficacy, Kwak et al [9] followed a total of 38 patients for 5 years. Compared to 6 months after the procedure, the net increase in the maximal glandular circumference was 14.10±0.65 mm but had decreased by 15% at 5 years. The mean grade of the patient's visual estimation was not significantly different between 6 months and 5 years. Furthermore, the percentage of patient satisfaction (Grs 3 and 4) did not differ significantly between the 6-month and 5-year follow-up. However, the mean grade of the patient's visual estimation was unchanged after 5 years compared with postoperative 6 months (Gr 3.60 vs. 3.56). This means that the patients might not have recognized the volume loss with the naked eye. A major advantage of HA gel over nonpermanent fillers, such as fat and collagen, is its increased tissue longevity. The slow digestion of this gel shows that stabilization of the material through cross-linkage is able to increase its longevity several hundred fold compared to a natural polymer, without decreased biocompatibility. The implant has the property of degradation, but it is isovolemic degradation. The isovolemic degradation keeps the gel always in balance with water in the tissue, and this increased capacity to bind water of a less concentrated HA network allows for the maintenance of the correction even in low concentrations of the material. Therefore, the gross appearance of the glans penis did not show any deformity at 5 years after augmentation in any of the patients. Despite the isovolemic degradation of HA supported by Q-Med, ultimately, time-dependent reabsorption can induce deformity that requires additional injection, but another advantage of HA gel is easy supplementation by reinjection in cases of long-term volume loss. Although manufacturers and several published articles claim that the fillers are non-toxic and non-immunogenic, or that complications are very uncommon [23], unwanted side-effects occur with all compounds used [162425]. In the early reports of HA injection for cosmetic purposes, no significant signs of bio-incompatibility were reported [2627]. Recent evidence may show that major, local and/or systemic, immediate or delayed adverse effects may appear in association with its use [28]. However, in this study, no serious adverse reactions, such as delayed and recurrent chronic inflammatory and granulomatous reactions, had occurred after 5 years of follow-up.
The current treatment choice for premature ejaculation is medical treatment. The main limitation of medical treatment for premature ejaculation is recurrence after withdrawal of medication. Hypersensitivity of the glans penis as a cause of premature ejaculation is still controversial, but many patients with primary premature ejaculation who respond to local anesthetics have penile hypersensitivity, which provides further support for an organic etiology of premature ejaculation [29]. Dorsal neurectomy can also be performed to decrease the sensitivity of the glans penis [30]. However, dorsal neurectomy is not an established treatment for penile hypersensitivity associated with premature ejaculation due to the technique's uncertain pathophysiology, as well as its invasiveness and side effects, for example, numbness, paresthesia, pain due to neuroma, Peyronie's disease, and even erectile dysfunction. Despite these limitations, dorsal neurectomy is still performed in selected patients who do not respond to conventional treatment for premature ejaculation.
The skin of the human phallus is innervated by the dorsal nerve of the penis (DNP). The main trunk of the DNP is composed of two different populations of axons [31]. The first group travels along the dorsal midline, terminating in the glans. The other group of fibers radiates from the main trunk over the lateral and ventral aspects of the penile shaft with branches to the corpus spongiosum and urethra. At 1 to 2 cm proximal to the corona glandis, the DNP dorsal trunk divides into two to three nerve bundles. The DNP and its branches along the shaft run just beneath the skin and fascia; the main branches within the glans are 3 to 6 mm from the epithelial surface. Halata and Munger [32] studied the sensory system of the human glans penis. The human glans penis is covered by stratified squamous epithelium and a dense layer of connective tissue equivalent to the dermis of typical skin. The papillary dermis blends into and is continuous with the dense connective tissue forming the tunica albuginea of the corpus spongiosum of the glans penis. The most numerous nerve terminals are free nerve endings present in almost every dermal papilla, as well as scattered throughout the deeper dermis. Genital bulbs are present throughout the glans, but are most numerous in the corona and near the frenulum. Moon's research groups (Moon et al [4], Kim et al [8], Kwak et al [9]) postulated the theoretical efficacy of GPA in premature ejaculation. Major contributing factors to the sensory characteristics of the glans penis are distribution of the dorsal nerve, number of receptors, threshold of receptors, and accessibility of stimuli to the receptors. Considering the studies of Yang and Bradley [31] and Halata and Munger [32], injectable implants can be successfully injected into the dermis of the glans penis just above the nerve terminal. Hence, the creation of a barrier by a bulking agent that inhibits tactile stimuli from reaching receptors may be effective in premature ejaculation by decreasing the sensation of the glans penis. Moreover, GPA is less harmful than invasive dorsal neurectomy.
Kim et al [8] compared the efficacy of GPA with dorsal neurectomy in a total of 139 patients with primary premature ejaculation. GPA with injectable HA gel was performed as they developed. The extent of nerve fibers, including in dorsal neurectomy, is important in postoperative sensation of the glans penis. To avoid excessive sensory loss, the dorsal branch on one side and ventral and lateral branches on the other side were excised in this study. At 6 months after each procedure, the volume effect using the same definition as in the GPA study, ejaculatory latency, the vibratory threshold of the glans penis using a biothesiometer (Bio Medical Instrument Co., Warren, MI, USA), the patient's satisfaction, and the partner's satisfaction were evaluated. In patients with GPA, the postoperative ejaculatory latency and vibratory threshold were significantly increased and 75% of patients were satisfied. In 10 of 74 patients with dorsal neurectomy, numbness (6), paresthesia (4), pain from neuroma (3), and Peyronie's disease (1) occurred, while no patients presented sensory loss among the 65 patients who underwent GPA. Abdallah et al [10] also reported increased intravaginal ejaculatory latency time (IELT) after 1 month of GPA using injectable HA gel. The IELT fell after 3 months but remained significantly higher than at baseline. Beginning in 2010, the International Society for Sexual Medicine (ISSM) Guidelines for the Diagnosis and Treatment of Premature Ejaculation classified the role of surgery, selective dorsal nerve neurectomy, and HA gel GPA as Level 4, that is, no evidence [33]. They do not recommend surgery, which may be associated with permanent loss of sexual dysfunction based on this study. However, this seems to be a mistake of the ISSM guideline committee in the interpretation of study results. Dorsal neurectomy patients have been reported to show a significant decrease in sensation measured by ejaculatory threshold and vibratory threshold (VT), but no permanent complications were reported in patients undergoing GPA alone. Compared to dorsal neurectomy, GPA has an additional benefit in patients with premature ejaculation without significant side effects or sexual dysfunction due to sensory loss.
Although, Moon's research groups (Moon et al [4], Kim et al [8], Kwak et al [9]) and Abdallah's research groups (Abdallah et al [10]) both demonstrated an increase in IELT after GPA and efficacy in selected patients with premature ejaculation, the major limitations of the treatment are invasiveness, side effects, and the possibility of further sensory loss over a longer period. To evaluate the long-term efficacy and side effects of GPA in PE, Kwak et al [9]. followed a total of 38 patients for 5 years. Compared to at the 6-month follow-up, IELT and VT were significantly lower at 5 years but still remained above baseline. Furthermore, the percentage of patient satisfaction at 5 years was just as high, at 76%, as it was at 6 months. Despite the bias in follow-up patients and some patients lost to follow-up, the patients who were satisfied at 6 months mostly remained satisfied at the 5-year follow-up.
The pathophysiology of premature ejaculation is poorly understood. The effects of GPA using filler in premature ejaculation might be the result of reduced sensation of the glans penis by formation of a barrier between stimuli and receptors, combined with increased self-confidence due to the subjective benefit of the procedure. To increase the efficacy of GPA by filler for premature ejaculation, proper patient selection is critical. As demonstrated in this study, the initial satisfaction rate at 6 months was maintained until 5 years despite the significant decrease in IELT and VT. The increased self-esteem and self-confidence from an enlarged glans may in itself have a positive effect. This means that proper patient selection and well-executed procedures can result in patient satisfaction.
After the development of GPA using injectable HA gel, various kinds of fillers have been used. In 2003, Perovic et al [6] reported his results with GPA by submucosal injection of hydrogel in 9 patients with glans deformity. He reported that hydrogel was safe and effective in 8 of the 9 cases for 12 to 26 months of follow-up, but he recommended simple aspiration for excessive hydrogel, if necessary. Shaeer [7] also used polyacrylamide gel injection for enhancing glans size in 2 patients following implantation of a penile prosthesis with satisfactory results, although the results were short-lived, requiring reinjection. The advantage of hydrogel is its lower price. However, polyacrylamide hydrogel has been shown to be a poor material for soft tissue augmentation in the long term. Hydrogel has been used in breast reconstruction. It has also been used for the correction of facial deformity. In 2003 and 2004, various complications of hydrogel, including diffuse paranasal swelling and signs of cellulitis from the granulomatous inflammatory response were reported, and the warning was that hydrogel injection is not appropriate for facial plasty [343536].
Despite of the proven safety of HA, with regard to both its unique characteristics and clinical outcomes, granulomatous foreign body reaction by protein contaminants and, rarely, ischemic necrosis can develop when injection is too superficial, too great a volume is injected, or intravascular injection occurs, causing immediate postinjection discoloration. Brody [37] and Hirsh et al [3839] reported the successful management of an unusual presentation of impending necrosis following a HA injection embolus and proposed an algorithm for management with hyaluronidase. In case of immediate postinjection discoloration, stopping the injection, applying gentle massage, and immediate application of heat and local nitroglycerine paste may be effective. If not improved, 75 U intradermal hyaluronidase injection (Vitrase; Ista Pharmaceuticals, Irvine, CA, USA) or 0.5 mL of 150 U hyaluronidase (50 U/mL; Lee Pharmacy Inc., Fort Smith, AZ, USA) will be effective in disappearance of 90% of the lumpiness in 24 hours and the remainder within the next few days. To avoid possible immediate ischemic necrosis, the use of a safe, appropriate filler and supplementation at postoperative 2 weeks instead of initial overinjection, and injection of hyaluronidase in case of HA gel are good alternatives.
Although not reported in the literature, several patients have been referred to us with complications (Fig. 4,5,6). The reason for local complications after filler injection are use of inadequate filler of unknown nature or poor purity and an incorrect injection technique such as too great a volume injected too superficially or misplaced injection, and local infection.
BellaGen (Hans Biomed Co., Seoul, Korea) is a suspension of modified SureDerm, cellular dermal fragments 500 to 1,000 mm in size, which is composed of a BellaGen 1 mL/5 mL syringe and 1.5 mL lidocaine+0.3 mL gentamycin/3 mL syringe. In case of discoloration after overinjection, it cannot be aspirated and hyaluronidase cannot digest the cellular fragments. Nowadays, various kinds of fillers have been developed. As illustrated in Fig. 7, some physicians favor fat injection, but surgical removal is very difficult even in successful cases if the patient is unsatisfied.
After development of GPA using injectable HA gel, Shaeer [40] reported a glans augmentation method by grafting which he named "Shaeer's glans augmentation". He also noted the need for GPA in patients lacking glans tumescence in penile prosthesis implantation or during natural erection and for a small glans after shaft augmentation. Shaeer's glans augmentation is insertion of a dermofat graft from the groin into the periurethral plane developed by dissection of a glans flap via two ventral incisions along the ventral aspect of the coronal sulcus.
In a pilot study of 10 patients, the maximum circumference of the glans increased by 16.6%, declining to a 14.2% increase at 10 to 12 months, which is a 2.3% decline. The self-reported impression of the augmented volume was high and well maintained over the follow-up period. Glans sensation, engorgement, erectile function, and ejaculatory control were preserved. The study reported promising results, with retention of the added volume at 1-year follow-up, preservation of sensitivity and engorgement, and no adverse effects on erectile function or ejaculatory control. Despite good results, major limitations of the study were invasiveness of the dermofat graft from the groin and lack of information on long-term results. Another similar method of GPA was reported in Korea but is unpublished.
A group of Korean andrologists developed an indirect GPA method. Compared to direct enhancement of the dermal layer of the glans penis in direct GPA by superficial injection of HA gel, indirect GPA involves implantation of a collagen scaffold of Lyoplant (B. Braun Melsungen AG, Melsungen, Germany) between the glans and corporal tip, which can produce general enlargement and elevation of the glans penis as illustrated in Fig. 8. As reported by Perovic et al [41], a potential space between the corpus spongiosum of the glans penis and the distal tip of the corpus cavernosum can be developed by blunt dissection via incision at the coronal sulcus. During preservation of the dorsal neurovascular bundle, the prepared commercial dermal strip, silastic bar, or dermis (autologous, AlloDerm [LifeCell Corporation, Branchburg, NJ, USA], Lyoplant) can be implanted. Simple closure and normal wound care is sufficient. At first, they also used a dermofat graft but changed to Lyoplant to avoid the invasiveness of the dermofat. Lyoplant implantation showed good results but resorption was a problem. This novel method appears to be very simple but the limitations are invasiveness, no proven efficacy, and a lack of other reports on the method.
Currently, GPA using injectable HA gel is not recommended in the ISSM guideline due to possible sensory loss. However, in a 5-year long-term follow-up of glans penis augmentation by subcutaneous injection of HA gel, the residual volume of implants decreased by 15% of the maximal glandular circumference, but was still effective for hypersensitivity of the glans penis in premature ejaculation patients. For efficacy in premature ejaculation, selection of appropriate patients is critical. GPA is not harmful to erectile function and is less invasive and irreversible than dorsal neurectomy. To refine the procedure, greater interest and proper studies are needed for the establishment of the procedure. GPA is an appropriate procedure for the relevant indications.
Figures and Tables
Fig. 1
At 6 months after hyaluronic acid gel injection into the dermis of the penis in the Beagle dog (H&E, ×200). (A) Normal control. (B) Implanted basophilic amorphous materials (Alcian blue stain). (C) Alcian blue stain after treatment with hyaluronidase reveals the digestion of hyaluronic acid gel.

References
3. Chew KK, Stuckey BG. Use of transurethral alprostadil (MUSE) (prostaglandin E1) for glans tumescence in a patient with penile prosthesis. Int J Impot Res. 2000; 12:195–196.

4. Moon DG, Kwak TI, Cho HY, Bae JH, Park HS, Kim JJ. Augmentation of glans penis using injectable hyaluronic acid gel. Int J Impot Res. 2003; 15:456–460.

5. Kim JJ, Kwak TI, Jeon BG, Cheon J, Moon DG. Human glans penis augmentation using injectable hyaluronic acid gel. Int J Impot Res. 2003; 15:439–443.

6. Perovic S, Radojicic ZI, Djordjevic MLj, Vukadinovic VV. Enlargement and sculpturing of a small and deformed glans. J Urol. 2003; 170(4 Pt 2):1686–1690. discussion 1690

7. Shaeer O. Supersizing the penis following penile prosthesis implantation. J Sex Med. 2010; 7:2608–2616.

8. Kim JJ, Kwak TI, Jeon BG, Cheon J, Moon DG. Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation. Int J Impot Res. 2004; 16:547–551.

9. Kwak TI, Jin MH, Kim JJ, Moon DG. Long-term effects of glans penis augmentation using injectable hyaluronic acid gel for premature ejaculation. Int J Impot Res. 2008; 20:425–428.

10. Abdallah H, Abdelnasser T, Hosny H, Selim O, Al-Ahwany A, Shamloul R. Treatment of premature ejaculation by glans penis augmentation using hyaluronic acid gel: a pilot study. Andrologia. 2012; 44:Suppl 1. 650–653.

11. Elson ML. Soft tissue augmentation. A review. Dermatol Surg. 1995; 21:491–500.
12. Pollack SV. Silicone, fibrel, and collagen implantation for facial lines and wrinkles. J Dermatol Surg Oncol. 1990; 16:957–961.

13. Olenius M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles, and folds. Aesthetic Plast Surg. 1998; 22:97–101.

14. Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg. 1998; 24:1317–1325.
15. Goa KL, Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994; 47:536–566.
16. Larsen NE, Pollak CT, Reiner K, Leshchiner E, Balazs EA. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993; 27:1129–1134.

17. Richter W. Non-immunogenicity of purified hyaluronic acid preparations tested by passive cutaneous anaphylaxis. Int Arch Allergy Appl Immunol. 1974; 47:211–217.

18. Richter AW, Ryde EM, Zetterström EO. Non-immunogenicity of a purified sodium hyaluronate preparation in man. Int Arch Allergy Appl Immunol. 1979; 59:45–48.

19. Gibbs DA, Merrill EW, Smith KA, Balazs EA. Rheology of hyaluronic acid. Biopolymers. 1968; 6:777–791.

20. Balazs EA. Intercellular matrix of connective tissue. In : Finch CE, Hayflick L, editors. Handbook of the biology of aging. New York: Van Nostrand Reinhold;1977. p. 22–240.
21. DeVore DP, Hughes E, Scott JB. Effectiveness of injectable filler materials for smoothing wrinkle lines and depressed scars. Med Prog Technol. 1994; 20:243–250.
22. Läckgren G, Wåhlin N, Stenberg A. Endoscopic treatment of children with vesico-ureteric reflux. Acta Paediatr Suppl. 1999; 88:62–71.

23. Engelman DE, Bloom B, Goldberg DJ. Dermal fillers: complications and informed consent. J Cosmet Laser Ther. 2005; 7:29–32.

24. Andre P, Lowe NJ, Parc A, Clerici TH, Zimmermann U. Adverse reactions to dermal fillers: a review of European experiences. J Cosmet Laser Ther. 2005; 7:171–176.

26. Bergeret-Galley C. Comparison of resorbable soft tissue fillers. Aesthet Surg J. 2004; 24:33–46.

27. Piacquadio D, Jarcho M, Goltz R. Evaluation of hylan b gel as a soft-tissue augmentation implant material. J Am Acad Dermatol. 1997; 36:544–549.

28. Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008; 22:150–161.

29. Xin ZC, Chung WS, Choi YD, Seong DH, Choi YJ, Choi HK. Penile sensitivity in patients with primary premature ejaculation. J Urol. 1996; 156:979–981.

30. Tullii RE, Guillaux CH, Vaccari R, Ferreira R. Premature ejaculation-selective neurectomy: A new therapeutic technique-base, indication and results. Int J Impot Res. 1994; 6:109–113.
31. Yang CC, Bradley WE. Neuroanatomy of the penile portion of the human dorsal nerve of the penis. Br J Urol. 1998; 82:109–113.

32. Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res. 1986; 371:205–230.

33. Althof SE, Abdo CH, Dean J, Hackett G, McCabe M, McMahon CG, et al. International Society for Sexual Medicine. International Society for Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med. 2010; 7:2947–2969.

34. de Bree R, Middelweerd MJ, van der Waal I. Severe granulomatous inflammatory response induced by injection of polyacrylamide gel into the facial tissue. Arch Facial Plast Surg. 2004; 6:204–206.

35. Kang SS, Zhang ZW, Chou HY, Zhai HF. Complication of polyacrylamide hydrogel injection for facial plasty. Zhonghua Zheng Xing Wai Ke Za Zhi. 2003; 19:325–327.
36. Liu Y, Cen Y, Xu XW, Duan WQ. Analysis of complications induced by polyacrylamide hydrogel injection for augmentation mammaplasty. Zhonghua Zheng Xing Wai Ke Za Zhi. 2005; 21:464–466.
37. Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005; 31:893–897.

38. Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007; 33:357–360.

39. Hirsch RJ, Brody HJ, Carruthers JD. Hyaluronidase in the office: a necessity for every dermasurgeon that injects hyaluronic acid. J Cosmet Laser Ther. 2007; 9:182–185.

41. Perovic SV, Scepanovic DR, Vukadinovic VM, Djakovic NG, Djordjevic ML. Penile disassembly technique: a new approach in the surgical reconstruction of hypospadias. Prog Urol. 1999; 9:371–379.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download