Abstract
Cryptorchidism is a well-known congenital anomaly in children. However, its diagnosis is often delayed for reasons including patient unawareness or denial of abnormal findings in the testis. Moreover, it has been difficult to establish an optimal treatment strategy for postpubertal cryptorchidism, given the small number of patients. Unlike cryptorchidism in children, postpubertal cryptorchidism is associated with an increased probability of neoplasms, which has led orchiectomy to be the recommended treatment. However, routine orchiectomy should be avoided in some cases due to quality-of-life issues and the potential risk of perioperative mortality. Based on a literature review, this study proposes individualized treatment guidelines for postpubertal cryptorchidism.
Cryptorchidism is a pathological condition in which the testis fails to descend to the base of the scrotum. It is one of the most common congenital anomalies encountered in pediatric urology. Despite extensive study, knowledge regarding the etiology and eventual consequences of cryptorchidism remains limited. Cryptorchidism occurs in 1% to 4% of full-term and 1% to 45% of preterm male neonates [1]. Its prevalence reaches approximately 1% by the age of one year, while relatively few cases are newly diagnosed in patients older than one year [2]. Treatment strategies for cryptorchidism in children are well-established. A surgical approach, most often orchiopexy, is recommended for testes that remain undescended after six months of age [3]. However, it has been difficult to establish a standard treatment for postpubertal cryptorchidism, given the small number of patients with this condition. Unlike cryptorchidism in children, postpubertal cryptorchidism is associated with an increased probability of neoplasms, which has led orchiectomy to be the recommended treatment [4]. However, routine orchiectomy should be avoided for several reasons. Therefore, we performed a literature review to identify individualized treatment guidelines for postpubertal cryptorchidism.
The diagnosis and treatment of postpubertal cryptorchidism and prepubertal cryptorchidism are markedly different processes. A physician encountering postpubertal cryptorchidism during an office examination should consider the risk of testicular cancer development, impacts on spermatogenesis, altered endocrine function, the patient's cosmetic appearance, the condition of the contralateral testis, and other factors including torsion, trauma, and associated hernia. The most important factor affecting mortality is the risk of testicular cancer. The second most important factor is fertility, which is most intensely affected by alterations in endocrine function and spermatogenesis.
The clinical importance of cryptorchidism is mainly due to the risk of testicular cancer, as approximately 10% of testicular tumors are believed to arise from cryptoid testes. The relative risk of neoplastic changes in undescended testes is 40 times higher than in descended testes [3]. The estimated rate of in situ carcinoma in patients with cryptorchidism is approximately 1.7% [5]. The effect of orchiopexy on testicular tumors remains controversial, although prepubertal orchiopexy may reduce the risk of tumor formation in undescended testes [6].
In a large cohort study of 16,983 men who were surgically treated for cryptorchidism, the risk of testicular cancer among those treated at 13 years of age or older was approximately twice that of men who underwent orchiopexy before the age of 13 years [7]. In a systematic review, the risk of testicular cancer in patients with cryptorchidism was found to be increased by 74% in the contralateral testis, in contrast to the more than six-fold increase in risk on the ipsilateral side [8]. The authors concluded that in cases of unilateral cryptorchidism, the risk of testicular cancer may be increased on both sides, although to a much greater extent on the ipsilateral side. The results indicate that both the ectopic position of the testis and shared risk factors are involved in the mechanism behind the association between cryptorchidism and testicular cancer.
Untreated cryptorchidism has been definitively associated with infertility. The development of postnatal germ cells deteriorates in the undescended testis after the first year, and perhaps for this reason, the risk of infertility increases with age [91011].
In a study of 767 boys with unilateral cryptorchidism who underwent orchidopexy and bilateral testicular biopsies between birth and nine years of age, gonocytes failed to disappear and adult dark spermatogonia failed to appear in the undescended testes in patients under one year of age, indicating a defect in the first step of testicular maturation at two to three months that resulted in the absence of an adequate adult stem cell pool [12]. In the same study, primary spermatocytes failed to appear in undescended testes and appeared in only 19% of contralateral descended testes at four to five years of age, indicating a defect in the initiation of meiosis.
In a study of 12 patients who underwent testis biopsies, all subjects with unilateral adult cryptorchidism had abnormal histologic findings [4]. Three of the four patients who underwent preoperative semen analysis showed abnormal findings. However, four of the six married patients had children. All of the patients with bilateral cryptorchidism showed abnormal findings in the histology of the testis and in semen analysis, and none had children.
Although Leydig cells are less vulnerable to damage, endocrine function is impaired in postpubertal cryptorchidism. Leydig cell hyperplasia is present in adults with uncorrected cryptorchidism [13]. Immunohistologic evidence of reduced functional Leydig cell activity is also present in such cases [14]. The assessment of the presence and function of Leydig cells in adults with cryptorchidism suggests that men with cryptorchidism do not have compromised Leydig cell function unless the intertubular connective tissue is damaged, which is associated with absent or altered Leydig cells [15].
A study investigated the endocrine function of patients with cryptorchidism to determine differences in the function of the Leydig cell-pituitary axis in formerly unilateral cryptorchid men [16]. The following variables were analyzed in subjects and in controls: luteinizing hormone; testosterone; free testosterone; follicle-stimulating hormone; inhibin B; sperm density, motility and morphology; testicular volume; and patient weight and age at orchiopexy or at the time of other childhood surgery (in controls). Men who underwent orchiopexy later in childhood displayed subclinically decreased Leydig cell function, which could potentially result in a hormonal milieu that would be less than optimal for adult reproductive function.
With regard to the overall endocrine function of patients with cryptorchidism, hormone replacement therapy can be considered after bilateral orchiectomy in cases involving bilateral nonpalpable testes.
Testes that have been in an undescended position for a significant number of years are unlikely to have the ability to produce functioning spermatogonia and pose a significant risk of developing a malignancy. Considerable uncertainty exists regarding the treatment of children or young adults who present late with cryptorchidism. Maldescent of the testis has been clearly shown to be an increased risk of malignant changes. Should these individuals be managed initially with orchiopexy, or should they proceed directly to orchiectomy?
Many authors have reported that testes that remain undescended until after puberty lose their functionality, and that fertility rates are not improved after postpubertal repair [171819]. Therefore, it has been recommended that the testes be resected in such patients because they cannot produce spermatozoa and have a significant risk of malignant changes. In other studies, orchiectomy was recommended even more emphatically, especially in unilateral cases, for reasons including abnormal findings in semen analysis and the presence of testicular neoplasms in follow-up examinations.
In contrast, some articles on the topic of postpubertal cryptorchidism have found postpubertal orchiopexy to be capable of leading to fertility by initiating spermatogenesis [202122]. Shin et al [22] reported the induction of spermatogenesis and pregnancy after adult orchiopexy. Lin et al [21] reported successful testicular sperm extraction and paternity in an azoospermic man after bilateral postpubertal orchiopexy.
In a series of 25 males (29 testes) presenting at a postpubertal age with cryptorchidism, orchiectomy was performed in 22 testes and orchiopexy for the remaining seven. The testes had been in an anomalous location for between 20 and 51 years. One of the testes was found to have a malignancy consisting of a pure seminoma. In the subsequent follow-up period, none of the patients in either group (orchiopexy or orchiectomy) developed a malignancy in the contralateral testis [23].
In another series of 81 patients presenting after the age of 14 years with either unilateral or bilateral cryptorchidism, two patients developed a testicular malignancy. Most of the cases (93%) had secretory azoospermia in association with seminiferous abnormalities and interstitial fibrosis [24].
In cases of postpubertal cryptorchidism, orchiectomy should be offered and, if declined, careful surveillance for malignant changes should be performed post-orchiopexy. If convincing evidence exists of descent at birth, with secondary ascent of the testis, postpubertal orchiopexy may reduce the risk of infertility without increasing the risk of malignancy [25].
The treatment options for postpubertal cryptorchidism include orchiectomy, orchiopexy, and observation with no operation. Generally, if the patient anticipates no beneficial effect on fertility and is expected to have an increased risk of cancer, orchiectomy is recommended. Moreover, testicular prostheses may lead to better cosmetic outcomes than a small testis. Orchiopexy may be recommended in light of the patient's endocrine functionality and the anticipation of fertility via intracytoplasmic sperm injection. However, in some cases, such as elderly patients with no further risk of cancer, observation with no operation may be considered, due to the risk of mortality associated with surgery.
In 1975, Martin and Menck [26] initially presented an analytical protocol for treating postpubertal males with cryptorchidism. They recommended performing orchiectomy in patients up to the age of 50 years major advances in germ cell therapy in the 1970s led to a significant increase in the survival rate of patients with testis germ cell malignancies in comparison to the data incorporated into Martin and Menck's recommendations [26]. A significant shift has taken place in attitudes towards managing adult cryptorchidism. Based on an analysis of a database of 34,135 orchiectomies that incorporated age, the American Society of Anesthesiologists (ASA) score, specific mortality data, and the relative risk of death from germ cell tumors, Farrer et al [27] recommended limiting surgical intervention to patients aged 32 years or younger. Motivated by advances in combination chemotherapy, Oh et al [28] analyzed mortality data on germ cell malignancies and data regarding perioperative mortality in orchiectomies in the United States from the National Center for Health Statistics. They concluded that the anesthetic and operative risk of surgery for an intra-abdominal testis in healthy adults up to the age of 50 years is so negligible that surgery should be offered to this group, and may also be considered in healthy ASA class I patients up to the age of 60 years.
If left untreated beyond the age of 11 years, abdominal cryptorchidism carries a 32 times higher risk of malignancy [29], as well as an increased risk of torsion. Spermatogenesis is virtually absent. This warrants an aggressive diagnostic and therapeutic approach [1727].
Traditionally, patients presenting with cryptorchidism have required either a groin exploration or laparotomy. These procedures have an associated risk of morbidity, increased postoperative analgesia requirements, problematically long convalescence periods, and produce chronically painful and disfiguring scars.
Laparoscopy with or without orchiectomy has been reported to be a safe and reliable procedure in adults with nonpalpable testes. It has been associated with minimal blood loss, short hospital stays, reduced analgesic requirements, and high patient satisfaction. Laparoscopy both provides an accurate diagnosis and enables definitive treatment. Laparoscopic evaluation and treatment have been recommended as the gold standard for maldescended testes in adults [30].
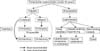
Laparoscopic evaluation and orchiectomy can be recommended in cases of postpubertal cryptorchidism with nonpalpable testes. Testosterone replacement therapy and artificial testis insertion can be recommended after bilateral orchiectomy in cases of bilateral nonpalpable testes (Fig. 1).
In the majority of cases, postpubertal cryptorchidism is associated with low fertility potential, impaired endocrine function, and an increased risk of testicular cancer. In patients under 50 years of age with a palpable testis and a normal contralateral testis, orchiectomy is the preferable treatment. In patients with a single testis or bilateral postpubertal cryptorchidism, preservative treatment may be considered, although such treatment requires careful follow-up. In postpubertal cryptorchidism with nonpalpable testes, the laparoscopic approach is recommended. Orchiectomy or careful observation may be considered in patients over 50 years of age with palpable cryptorchidism. After orchiopexy, patients should regularly perform self-examinations of the testes over the entire course of their lives.
Figures and Tables
ACKNOWLEDGEMENTS
This work was supported by a 2015 Pusan National University Yangsan Hospital research grant.
References
1. Sijstermans K, Hack WW, Meijer RW, van der Voort-Doedens LM. The frequency of undescended testis from birth to adulthood: a review. Int J Androl. 2008; 31:1–11.

2. Moradi M, Karimian B, Moradi A. Adult orchidopexy: A survey on necessity of intraoperative testicular biopsy. Nephro Urol Mon. 2011; 3:196–200.
3. Barthold JS. Abnormailities of the testis and scrotum and their surgical management. In : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 10th ed. Philadelphia: Saunders;2012. p. 3560–3574.
4. Jeong SC, Lee S, Ku JY, Lee SD. Clinical characteristics and treatment of cryptorchidism in adults: a single center experience. World J Mens Health. 2014; 32:110–115.

5. Giwercman A, Bruun E, Frimodt-Møller C, Skakkebaek NE. Prevalence of carcinoma in situ and other histopathological abnormalities in testes of men with a history of cryptorchidism. J Urol. 1989; 142:998–1001.

6. Coupland CA, Chilvers CE, Davey G, Pike MC, Oliver RT, Forman D. United Kingdom Testicular Cancer Study Group. Risk factors for testicular germ cell tumours by histological tumour type. Br J Cancer. 1999; 80:1859–1863.

7. Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med. 2007; 356:1835–1841.

8. Akre O, Pettersson A, Richiardi L. Risk of contralateral testicular cancer among men with unilaterally undescended testis: a meta analysis. Int J Cancer. 2009; 124:687–689.

9. American Academy of Pediatrics. Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychopsychological effects of surgery and anesthesia. Pediatrics. 1996; 97:590–594.
10. Hutson JM, Hasthorpe S. Abnormalities of testicular descent. Cell Tissue Res. 2005; 322:155–158.

12. Huff DS, Fenig DM, Canning DA, Carr MG, Zderic SA, Snyder HM 3rd. Abnormal germ cell development in cryptorchidism. Horm Res. 2001; 55:11–17.

13. Gotoh M, Miyake K, Mitsuya H. Leydig cell hyperplasia in cryptorchid patients: quantitative evaluation of Leydig cells in undescended and contralateral scrotal testes. Urol Res. 1984; 12:159–164.

14. Regadera J, Codesal J, Paniagua R, Gonzalez-Peramato P, Nistal M. Immunohistochemical and quantitative study of interstitial and intratubular Leydig cells in normal men, cryptorchidism, and Klinefelter's syndrome. J Pathol. 1991; 164:299–306.

15. Romeo R, Marcello MF. Some considerations on the human Leydig cell (immunohistochemical observations). Arch Ital Urol Androl. 1999; 71:143–148.
16. Lee PA, Coughlin MT. Leydig cell function after cryptorchidism: evidence of the beneficial result of early surgery. J Urol. 2002; 167:1824–1827.

17. Rogers E, Teahan S, Gallagher H, Butler MR, Grainger R, McDermott TE, et al. The role of orchiectomy in the management of postpubertal cryptorchidism. J Urol. 1998; 159:851–854.
18. Grasso M, Buonaguidi A, Lania C, Bergamaschi F, Castelli M, Rigatti P. Postpubertal cryptorchidism: review and evaluation of the fertility. Eur Urol. 1991; 20:126–128.

19. Okuyama A, Nonomura N, Nakamura M, Namiki M, Fujioka H, Kiyohara H, et al. Surgical management of undescended testis: retrospective study of potential fertility in 274 cases. J Urol. 1989; 142:749–751.

20. Heaton ND, Davenport M, Pryor JP. Fertility after correction of bilateral undescended testes at the age of 23 years. Br J Urol. 1993; 71:490–491.

21. Lin YM, Hsu CC, Wu MH, Lin JS. Successful testicular sperm extraction and paternity in an azoospermic man after bilateral postpubertal orchiopexy. Urology. 2001; 57:365.

22. Shin D, Lemack GE, Goldstein M. Induction of spermatogenesis and pregnancy after adult orchiopexy. J Urol. 1997; 158:2242.

23. Granados Loarca EA, Esau Ortega S. Is necessary to practice orchiectomy in patients with post-puberal maldescended testes? Actas Urol Esp. 2005; 29:969–973.
24. Ben Jeddou F, Ghozzi S, Rais NB. Cryptorchidism in adults. About 81 cases. Tunis Med. 2005; 83:742–745.
25. Hutson JM, Clarke MC. Current management of the undescended testicle. Semin Pediatr Surg. 2007; 16:64–70.

27. Farrer JH, Walker AH, Rajfer J. Management of the postpubertal cryptorchid testis: a statistical review. J Urol. 1985; 134:1071–1076.

28. Oh J, Landman J, Evers A, Yan Y, Kibel AS. Management of the postpubertal patient with cryptorchidism: an updated analysis. J Urol. 2002; 167:1329–1333.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download