This article has been corrected. See "Corrigendum: Testosterone Replacement Therapy and Cardiovascular Risk: A Review" in Volume 34 on page 154.
Abstract
Recent reports in the scientific and lay press have suggested that testosterone (T) replacement therapy (TRT) is likely to increase cardiovascular (CV) risk. In a final report released in 2015, the Food and Drug Administration (FDA) cautioned that prescribing T products is approved only for men who have low T levels due to primary or secondary hypogonadism resulting from problems within the testis, pituitary, or hypothalamus (e.g., genetic problems or damage from surgery, chemotherapy, or infection). In this report, the FDA emphasized that the benefits and safety of T medications have not been established for the treatment of low T levels due to aging, even if a man's symptoms seem to be related to low T. In this paper, we reviewed the available evidence on the association between TRT and CV risk. In particular, data from randomized controlled studies and information derived from observational and pharmacoepidemiological investigations were scrutinized. The data meta-analyzed here do not support any causal role between TRT and adverse CV events. This is especially true when hypogonadism is properly diagnosed and replacement therapy is correctly performed. Elevated hematocrit represents the most common adverse event related to TRT. Hence, it is important to monitor hematocrit at regular intervals in T-treated subjects in order to avoid potentially serious adverse events.
Testosterone (T) preparations have been available for more than 70 years [123], but the use of costlier new preparations has expanded dramatically over the last two decades [4]. Data extracted in May 2014 from the Symphony Healthcare Solutions Anonymous Patient Longitudinal Database indicate that 2.2 million USA citizens were prescribed T in 2013, compared to 1.2 million in 2010 [5]. Androgen prescribing patterns in the USA have also been investigated using Clinformatics Data Mart, which is the nation's largest healthcare claims database. That study was focused on data from the last decade and involved more than 10 million men age 40 years or older. In this large cohort, T or other androgen prescriptions increased more than threefold, from 0.81% in 2001 to 2.91% in 2011 [6]. Interestingly, at least one out of four androgen users did not have a T level measurement taken in the previous 12 months, because primary care physicians were likely to write a prescription without first ordering a blood test. Finally, very recently, Muram et al [7] reported data on more than 63,000 men from the Truven Health Marketscan® Commercial and Medicare Supplemental Insurance Database collected between January 2010 and June 2012, obtaining virtually the same findings. In this large cohort, 71% of men had their T tested once, 40% twice, and 29% had no T measurements before T replacement therapy (TRT). Only 12% had their gonadotropins measured before the initiation of TRT. Following therapy, 46% of men had their T measured at least once.
It is reasonable to believe that the aggressive direct-to-consumer marketing strategy of USA pharmaceutical companies has resulted in this marked increase in T use, even without an appropriate diagnosis. In fact, media advertising promises that treating 'low T' can make men feel more alert, energetic, mentally sharp, and sexually functional. In fact, many men have obtained prescriptions not as a medical therapy for male hypogonadism, but as a way to combat fatigue, low sex drive, and weight gain, with the goal of regaining the vitality of their youth.
While T sales have soared, recent reports in the scientific and lay press have significantly curbed the enthusiasm for androgen boosting, suggesting that TRT increases cardiovascular (CV) risk. Based on these scientific and media reports, some nonprofit groups petitioned the USA Food and Drug Administration (FDA) to require warnings for CV risks on the drugs' packaging. In fact, one of the main missions of regulatory agencies is to evaluate the balance between the benefits and risks of medicinal products. This balance should be evidence-based and expressed in a transparent manner using a framework that aids in the communication of the differences in opinions between regulators and pharmaceutical companies. In a final report released in 2015, the FDA cautioned that prescribing T products is approved only for men who have low T levels caused by certain medical conditions [8]. In particular, only subjects with primary or secondary hypogonadism resulting from problems within the testis, pituitary, or hypothalamus (e.g., genetic problems or damage from surgery, chemotherapy, or infection) should be treated. In contrast, the FDA emphasized that the benefits and safety of T medications have not been established for the treatment of low T levels due to aging, even if a man's symptoms seem related to low T. Therefore, a labelling change to inform patients about the possible increased risk of heart attack and stroke was required. Is this statement evidence-based?
The aim of the present review was to examine the available evidence on the association between TRT and CV risk. It is important to note that well-designed, placebocontrolled trials of TRT in men who meet or do not meet the standard criteria for treatment are scant in number, and their results are often inconsistent. In addition, few randomized controlled trials (RCTs) having CV events as a primary endpoint are currently available, as the majority of information has been derived from trials designed for other purposes. Considering that additional information can also be obtained from observational and pharmacoepidemiological investigations, such studies were also scrutinized. However, observational clinical research suffers from important biases, including selection, information, and confounding biases, and caution is needed when evaluating results reporting small differences [9]. In addition, this type of study often suffers from the limitation of not being able to determine if subjects in the treatment arm took the medication at an adequate dosage.
In order to overcome, at least in part, all the aforementioned limitations, in this review we used already published meta-analyses as well as presenting some novel meta-analytic findings. Systematic reviews and meta-analyses are often considered to be the highest level of evidence for evaluating interventions in healthcare and to be particularly useful tools when addressing questions for which multiple sources of data conflict, or when a variety of reports with low statistical power have been published, because aggregating information can improve the power and lead to a more convincing result. In addition, combining results from different studies helps to identify patterns among study results, sources of disagreement among those results, and other interesting relationships that may become evident in the context of multiple studies.
To date, long-term prospective interventional RCTs on TRT with CV events as the primary, prespecified endpoint in patients with coronary heart disease (CHD) are scarce. Results are only available from six short-term studies (mean, 23 weeks). All of these studies enrolled a small number of patients with CHD who were treated with different formulations and doses of TRT (n=128) or placebo (n=129) [10]. Combining the results of these trials in a meta-analysis, TRT was positively associated with a significant increase in treadmill test duration (168 seconds) and time to ST segment depression (57 seconds) [10]. Table 1 summarizes the main characteristics of these studies [111213141516].
Similarly, the available studies of patients with heart failure (HF) are few and involve a short follow-up duration, but all available studies have reported significant improvements in exercise capacity after 12 to 52 weeks of TRT. The meta-analysis of four RCTs that included subjects with HF indicated a significant increase in exercise capacity of almost 54 meters using the six-minute walk test [17]. The authors commented that the effect of T was impressive and greater than that seen with other cardiologic therapies routinely recommended to patients with HF [17].
In contrast to the trials with CV events as the primary endpoint, which supported a protective effect of TRT on myocardial ischemia (MI) and improvement in HF, data from trials designed for other purposes have generated recent concerns about the CV safety of TRT. Therefore, meta-analytic surveys assessing the occurrence of adverse CV events in men treated with T or placebo in RCTs designed for other purposes have often been viewed with particular interest. Of the five available meta-analyses [1819202122], four [18192021] did not find either a protective or harmful effect of TRT on CV events, whereas one [22] suggested a possible increased risk associated with T treatment. Table 2 summarizes the main characteristics of the available meta-analyses. These meta-analyses were performed several years apart, thus including different sets of available studies: 2004 and earlier [1819], 2008 [20], 2012 [22], and 2014 [21]. In addition, the meta-analyses differed in the criteria for trial inclusion, particularly with regard to age limits and study duration (Table 2). More importantly, the outcomes of interest that were retrieved and analyzed differed across the meta-analyses (Table 2). In this study, we analyzed the results according to the main outcomes related to CV diseases (CVD).
As stated before, in the absence of any long-term prospective studies, the analysis of adverse CV events in RCTs might give insights into the potential risk associated with TRT. Five systematic reviews are currently available, and their results are summarized in Table 2. As shown in Table 2, the only meta-analysis reporting an increased CV risk associated with TRT was published two years ago in BMC Medicine by Xu et al [22]. The authors selected studies lasting 12 weeks or more that clearly reported all CV-related events (Table 2). No clarifications were provided about the articles that were excluded. Among 1,882 studies, 27 trials, including 2,994 men, were selected and meta-analyzed. The primary outcome was composite CV events, defined as anything reported as such by the study's authors. A forest plot indicated that T increased the risk of CV events, with an odds ratio (OR) of 1.54 (95% confidence interval [CI]=1.09~2.18), which was even higher (OR=2.06 [95% CI=1.34~3.17]) when the results were categorized according to the presence of pharmaceutical industry funding. The construct of composite CV-related events included all investigator-reported adverse events affecting the CV system. Hence, cases of peripheral edema and self-reported syncope were also included in the category of CV events, leading to an artificial increase of the overall number of events. The overly broad definition of CV endpoints, in which some endpoints were inappropriately reported as CV-related by the investigators, increased the statistical power of the analysis, but may have been grossly misleading due to the heterogeneity and limited reliability of diagnostic criteria used to classify these events as drug-related. In addition, all analyses of all CV events classified as 'serious' (including all instances of hospitalization) inherently have the limitation of including in the endpoints many events that are investigator-driven, such as revascularization. In our opinion, the assessment of the CV safety of any therapy should be based on the incidence of major adverse cardiac events (MACE), which are easier to detect and less controversial to diagnose.
Nonetheless, misclassification may occur due to undefined screening procedures and diagnostic criteria, unless trials are specifically designed to assess CV outcomes. In the meta-analysis of Xu et al [22], the Basaria et al's [23] trial, with the highest overall weight, was clearly discordant from all the others. The Basaria et al's [23] study likewise used a very broad definition of CV events. In that RCT, the authors randomized men aged 65 years or older (mean age, 74±5 years) with limitations in mobility and total T levels between 3.5 and 12.1 nmol/L or free T <173 pmol/L to placebo or a supraphysiological dose of T gel (100 mg daily) for six months, in order to assess the effect of TRT on exercise tolerance. The cohort consisted of elderly community-dwelling men with a high prevalence of hypertension, obesity, diabetes, dyslipidemia, and known CVD. Although improved physical function was noted (including leg- and chest-press strength and stair climbing while carrying a load) the trial was ended early due to imbalances in respiratory, dermatological and, most importantly, CV events between the two arms (23 CV events in the T group vs. five in the placebo group). However, the diversity of the cardiac events that occurred and the lack of their structured classification strongly limited the clinical value of the study. Moreover, the population studied (frail men) and the utilization of high T dosages (double the recommended dose) further limit the study's generalizability. It is interesting to note that the majority of adverse CV events were reported in men with T blood levels above 34.6 nmol/L. It is possible that the increased vigor in the T arm allowed previously frail men to engage in more strenuous physical activities, therefore unmasking pre-existing CVD. Overall, this study suggested caution in prescribing TRT therapy for aging frail men, especially if they have symptomatic CVD.
The Xu et al's [22] meta-analysis did not agree with the previous meta-analyses [18192021]; in fact, all the other meta-analyses showed no significant difference between the T and placebo groups for the incidence of all CV events or for each type of event (CV death, fatal and non-fatal MI, revascularization procedures, arrhythmia, and cerebrovascular events), except the aforementioned increase in hematocrit over 50%, which was significantly more prevalent in the T group. In 2005, Calof et al [18] published the first meta-analysis on this topic, reviewing adverse events in 19 studies performed between 1966 and 2004. They reported 18 CV events in the 651 T-treated men and 16 events in the 433 placebo-treated men, with a pooled OR of 1.14 (95% CI=0.59~2.20). Haddad et al [19] scrutinized 30 placebo-controlled studies conducted between 1966 and 2004 for possible T-induced adverse effects and meta-analyzed six studies that reported CV events. Fourteen events were observed among the 161 men treated with T and seven events were observed among the 147 men in the placebo arm. Finally, Fernández-Balsells et al [20] meta-analyzed 51 placebo-controlled studies conducted from 2003 to 2008, with follow-up ranging from three months to three years. They found no significant differences in the rates of death, MI, revascularization procedures, or cardiac arrhythmias between the T and the placebo groups.
We recently performed the last updated systematic review and meta-analysis of RCTs of TRT, using a more conventional definition of CV events similar to that used by regulatory authorities to verify the safety of newly registered drugs [21]. We included all RCTs that enrolled men, compared the effect of TRT against placebo on different endpoints, and provided information on CV-related events by study arm without any arbitrary restriction, even if CV events were not the principal endpoints of the study. The principal outcome evaluated in this analysis was the effect of TRT, as compared to placebo, on the incidence of MACE. The MACE endpoint was defined as the composite of CV death, non-fatal MI and stroke, and acute coronary syndromes and/or HF reported as serious adverse events [21].
Secondary outcomes included all CV-related events, defined as anything reported as such by the authors of the individual studies. In a meta-analysis of the largest number of studies collected so far (Table 2), we (Corona et al [21]) did not observe any increase in CV risk associated with TRT when either composite or single CV endpoints were considered. In particular, the use of TRT was not associated with an increased incidence of MACE compared to placebo (Mantel-Haenzel OR=1.01 [95% CI=0.57~1.77]; p=0.98). In addition, meta-regression analysis showed no difference in the incidence of MACE according to baseline age, body mass index, or level of T (S=0.03 [95% CI=-0.04~0.10]; p=0.40, S=-0.07 [95% CI=-0.29~0.14]; p=0.51; S=-0.14 [95% CI=-1.17~0.89]; p=0.79, respectively). Since the OR for MACE with TRT was 1.01 [95% CI=0.57~1.77], the data are sufficient only to exclude any increase of risk greater than 77%. It is important to note that the duration of the available trials scrutinized was relatively short. Therefore, although no clear signs of short-term risk were noted, no information is available on possible long-term effects. Another limitation of the analysis is the incomplete reporting of the data on MACE in trails marginally designed for non-CV endpoints. However, the use of MACE, instead of a broader definition of CV side effects, has the advantage of a clearer diagnostic definition, which is less dependent on investigators' subjective opinions. In fact, although the criteria for the diagnosis of MI or stroke could also be questioned, such entities are much more clearly defined than 'peripheral edema' or 'self-reported syncope,' which have been included as CV events in some other analyses [2223]. Interestingly, regulatory agencies assessing the safety of drugs require analyses of MACE and not of broadly defined CV side effects.
It is important to note that when our meta-analysis was categorized according to the baseline study population characteristics, a protective role of TRT in subjects with metabolic disease was observed [22]. This is surprising because, by meta-analyzing the available evidence, we previously demonstrated an association between TRT and improvements of fat mass and glycometabolic control in subjects with type 2 diabetes and metabolic syndrome [2425]. Similar results have been more recently confirmed by other authors [26]. However, the number of RCTs evaluating the effect of TRT in subjects with metabolic disease is too limited to draw any conclusions. In contrast, no difference between TRT and placebo was detected in other subpopulations, including subjects with previous CVD and frail men. Finally, in contrast to the findings of Xu et al [22], we (Corona et al [21]) did not observe any difference in the CV risk even when the analysis was categorized according to the presence or absence of drug company support.
Erythrocytosis is an increase in the total red cell mass secondary to any of a number of non-hematologic systemic disorders in response to a known stimulus (secondary polycythemia), in contrast to primary polycythemia (polycythemia vera). Erythrocytosis, per se, should not be considered a true CV event. However, some reports have shown that a higher risk of MI or was stroke associated with T-induced polycythemia resulting from intermittent high-dose T administration [27]. In addition, pre-existing erythrocytosis constitutes a risk factor for thrombosis in hypogonadal men [27].
Erythrocytosis is the most frequent adverse event associated with T administration in clinical trials, as well as in clinical practice. T is an important regulator of red blood cell production through the augmentation of erythropoietin and its direct effect on bone marrow, including erythroid progenitors, ferrokinetics, and red cell precursor survival. Recently, the suppression of circulating and hepatic hepcidin, an iron regulatory peptide, has been proposed as an additional mechanism of action of T on red cell mass [28]. Two meta-analyses have reported that T-treated subjects were three to four times more likely than those in the placebo arm to develop a hematocrit level >50% [1819]. A more recent meta-analysis reviewing all RCTs comparing the effect of TRT vs. placebo or the effect of TRT as a supplement to phosphodiesterase-type 5 inhibitors (PDE5is) on sexual function [29], confirmed that TRT was associated with an overall significant increase in hematocrit levels (standardized mean=0.899 [95% CI= 0.718~1.061]; p=0.0001). However, TRT did not increase the risk for pathological hematocrit levels (above the study reference levels) when compared to placebo (p=0.305). A recent meta-analysis of studies involving the long-lasting injectable T preparation (T undecanoate) confirmed increased hematocrit levels in some subjects but did not find any association with pathological erythrocytosis [30]. Similarly, by reviewing the data of our recent meta-analysis on the effect of TRT vs. placebo on CVD [21], we confirmed that TRT was associated with a significant increase of hematocrit in comparison to placebo as well as with an increased risk of levels above 52% (Fig. 1). However, when the data were limited to the studies enrolling only hypogonadal (T<12 nmol/L) patients at baseline treated with transdermal preparations, a risk of elevated hematocrit (>52%) was not found (hazard ratio [HR]=4.89 [95% CI=0.83~28.91]; p=0.08]). Accordingly, erythrocytosis has been reported to occur in healthy older men more often after short-action parenteral and oral T administration, but usually not after transdermal T administration [23].
Nonetheless, it is important to monitor hematocrit at regular intervals in order to avoid potentially serious adverse events [23]. Guidelines indicate that a hematocrit level above 54% is an indication to stop TRT until the hematocrit decreases to a safe level [313233]. Phlebotomy can also be considered in the most severe cases.
Pharmacoepidemiology is an observational bridge science, spanning epidemiology and clinical pharmacology. It studies the uses and effects of drugs in a well-defined population, with the aim of providing a risk-benefit assessment in large numbers of people by estimating the probability of beneficial effects of a drug and the probability of adverse effects. Pharmacoepidemiology is often based on large healthcare utilization databases using the non-experimental study of intended and unintended drug effects outside of RCTs, comparing events in an exposed group in comparison with a control (unexposed) group. Pharmacoepidemiological studies have led to important discoveries, such as the positive effect of aspirin on MI [34]. The strength of pharmacoepidemiology over RCTs is the ability to quantify adverse events that may occur over long periods. A total of six pharmacoepidemiological studies have been published with TRT-associated all-cause mortality and/or CV events as endpoints.
A retrospective observational study conducted in Seattle, WA, USA evaluated the mortality rate in a series of 1,031 T-treated hypogonadal male veterans 40 years or older, compared with untreated veterans with hypogonadism (total T<8.7 nmol/L) [35]. Over a mean follow-up period of 40.5 years, it was found that men receiving TRT (n=398) had a 39% decrease in mortality (HR=0.61 [95% CI=0.42~0.88]) in comparison to their untreated counterparts [35]. Similar results have been reported in another retrospective study on type 2 diabetic subjects, collecting data from outpatient medical facilities with access to medical records [36]. Over a mean follow-up of 5.8 years, men with a low T level (<10.4 nmol/L) had a two-fold increased risk of death in comparison with the rest of the diabetic population. Both studies used time-to-event Cox regression analysis. In apparent contrast with the aforementioned findings, another pharmacoepidemiological study was published in the Journal of the American Medical Association reporting data on mortality [37]. Vigen et al [37] retrospectively evaluated a cohort of 8,709 veterans who had undergone coronary angiography between 2005 and 2011 with low T levels (T <10.4 nmol/L). Some of the men received TRT, while others did not. Among the men who received any form of TRT, 25.7% had MACE or died from any cause, vs. 19.9% of those who did not receive hormonal therapy, with an HR of 1.29 (95% CI=1.04~1.58; p=0.02), which was not substantially affected by adjusting for confounders [37]. It should be noted that in this study T-associated mortality was not analyzed individually, but combined in a composite index with the risk of MI or stroke.
Although the study had many flaws, due to its retrospective design and deficiencies in the information available in the Veterans Administration database, it received considerable attention in the lay media. In the meantime, many critiques of this study have been made, particularly with respect to the lack of repeated T measurements and to the possibility of inadequate treatment in many of the examined cases. In fact, after a mean of 3.3 measurements, T levels rose from 175 ng/dL to 332 ng/dL, suggesting that the TRT in many, if not the majority, of men in that study was insufficient. These concerns, in the form of letters to the editor, were published in the same journal and were only partially addressed by the authors' rebuttal [38]. One of the main criticisms of the study was that 1,132 men who had received a T prescription after experiencing a CV event (MI or stroke, 36) were excluded from the study. Quite unexpectedly, the author's answer indicated the use of 'an incorrect notation' regarding this value [39]. In particular, they asserted that the number of men excluded for this reason was 128, not 1,132, after the data revision. This is an 89% error rate, involving more than 1,000 individuals. In addition, 100 women were erroneously included in the original group of 1,132 individuals.
Many of the pitfalls of the Vigen et al's [37] report were recently addressed by Sharma et al [40] in a study including the largest cohort and the longest follow-up to date. A total of 83,010 veterans with documented low T levels were retrospectively evaluated. The investigators delineated three groups: veterans who had (n=43,931; group 1) or had not (n=25,701; group 2) achieved 'normal' T levels (according to the individual laboratories) and untreated hypogonadal veterans (n=13,378; group 3). Using a propensity score-weighted Cox proportional hazard model, the group treated adequately with T (defined by achieving 'normal' T levels under treatment) had a significant reduction of all-cause mortality as well as MI and stroke. Furthermore, all-cause mortality and the risk of MI and stroke were lower in group 1 than in group 2, leading the study investigators to suggest that "patients who failed to achieve the therapeutic range after TRT did not see a reduction in MI or stroke."
By meta-analyzing these studies [35363740], we found that the lack of TRT in otherwise hypogonadal subjects increased their risk of mortality by 30%, although this finding did not reach statistical significance (p=0.07; Fig. 2A). In the present meta-analysis, we also considered the Vigen et al's [37] study, which, as stated before, did not individually report the data on mortality, but computed them together with MI and stroke. By performing a sensitivity analysis excluding the latter study, lack of TRT was clearly associated with a statistically significant increased risk of mortality (OR=1.50 [95% CI=1.25~1.80]; p <0.0001). Nonetheless, these results must be interpreted cautiously because residual confounding factors may still be a source of bias, including the substantial risk of a primary selection bias due to the nonrandom assignment of T exposure. Physicians often prefer to treat healthier individuals, and healthier individuals more often request treatment for their hypogonadism-related (sexual) problems, thus accounting for mortality being lowest in this group. Finally, three out of four studies used only all-cause mortality as the outcome, making it impossible to capture the extent of CV-related mortality.
A pharmacoepidemiological survey conducted at the University of Texas Medical Branch at Galveston (USA), examined 25,420 Medicare beneficiaries 66 years or older treated with T for up to eight years [41]. The authors selected 6,355 patients who were treated with at least one injection of T between 1997 and 2006 or until they lost Medicare coverage. Overall, they represented a sample of 5% of beneficiaries. The subjects in this cohort were matched to 19,065 T nonusers at a 1:3 ratio based on a composite MI prognostic score and were tracked until they enrolled in a health maintenance organization, experienced a MI, or died. The study found that TRT was not linked with any increased risk for MI (HR=0.84 [95% CI=0.69~1.02]). In contrast, men at greater risk for heart problems who underwent TRT actually had a lower rate of heart attacks than similar men who did not receive this treatment. Cox regression analysis, after adjusting for demographic and clinical characteristics, found an interaction between receiving T and the quartile of risk of MI (p=0.023). For men in the highest quartile of the MI prognostic score, TRT was associated with a reduced risk of MI (HR=0.69 [95% CI=0.53~0.92]), whereas no difference in risk was found for the first quartile (HR=1.20 [95% CI=0.88~1.67]), second quartile (HR=0.94 [95% CI=0.69~1.30]), and the third quartile (HR=0.78 [95% CI=0.59~1.01]). Hence, T injection lowered the risk of heart attack by approximately 30% in the group of men judged most likely to have a heart attack based on other factors. A dose-response analysis demonstrated no increased risk in MI according to the estimated cumulative dose of T. These findings were robust across a range of sensitivity analyses that addressed eligibility criteria, exposure thresholds, follow-up periods, and covariate adjustment [41].
Another retrospective study funded by the National Institutes of Health investigated, in a large healthcare database from Truven Health Analytics, the rate of nonfatal MI in 56,000 middle-aged and older men who were prescribed TRT [42]. The authors compared the rate of CV events in the 90 days after starting TRT with the rate over the prior year. The study reported a doubled risk of heart attack among men aged 65 years and older and a two- to three-fold increased risk in younger men with a pre-existing history of heart disease, but not in those without prior CV events. For comparison, the authors analyzed 167,000 individuals who were prescribed a PDE5i under the same conditions, and no increase in CV events was observed. The PDE5is were selected as a comparator arm because many indications for use are similar to T therapy and these agents are not associated with an increased risk of CV events. When they followed up men who underwent TRT for another 90 days, the risk declined to the level it was at the study's entry point among men who did not refill their initial prescription. The analysis offers the advantage of using more stringent criteria for CV events, as compared to the vague composite groups used in previous studies [43], and also correlated the novel incident events with the early phase of TRT. Although the authors found an association between T therapy and an increased risk of heart attack, the comparison of TRT with PDE5is is questionable because PDE5is have distinct cardioprotective effects.
A Canadian group from the University of British Columbia recently performed a case-control study within a cohort of 934,283 men aged 45 to 80 years, equally spread demographically throughout the USA, with data drawn from the IMS LifeLink Health Plan Claims Database. In this study, four controls were identified for each case of MI, using density-based sampling, and were followed-up for 10 years (2001~2011 [43]). Rate ratios were computed for current and past TRT patients. As a sensitivity analysis, the risk of MI before and after the start of a first-time TRT prescription in the same patient was also performed. The results demonstrated a 41% increased risk for MI in men who received TRT for the first time. However, a relationship between MI and past or current TRT was not found, and current use of T was not associated with an increased risk of MI (rate ratio=1.01, 95% CI=0.89~1.16). The consensus of this study was that no association was found between adverse CV events and continuing TRT [43].
By combining the results from these three studies [414243] with those of Sharma et al [40] and Vigen et al [37], we performed a meta-analysis with MI odds as the outcome. The results are presented in Fig. 2B. The rate ratio of MI lies exactly on the unity point, suggesting no relationship between any use of TRT and the risk of MI. Similar results were observed after the exclusion of Vigen et al [37] (HR=0.93 [95% CI=0.74~1.19]; p=0.57).
T exerts a variety of effects on blood vessels and the heart [242744]. The pathophysiology underlying potential associations between T and the CV system is rather complex, as the hormone has differing effects according to its binding to different receptors (androgen, estrogen, membrane-bound, and cytosolic receptors) and interindividual variability. The best documented effect of TRT is the stimulation of red blood cell production [2427]. Hence, it is important to monitor hematocrit at regular intervals in T-treated subjects in order to avoid potentially serious adverse events. Previous and present meta-analyses of the available evidence on RCTs, whether or not they had CV events their primary endpoint, and pharmacoepidemiological studies do not support any causal role between TRT and adverse CV events. This is especially true when hypogonadism is properly diagnosed and replacement therapy correctly performed. The misdiagnosis and overtreatment of hypogonadism could be related to an increased risk, as shown by the Basaria et al's [23] study. However, as demonstrated elsewhere [24444546], TRT might represent an important new strategy in improving cholesterol and glucose levels and reducing body fat and increasing lean muscle mass, which are all factors that reduce the risk of heart disease. Accordingly, by meta-analyzing studies involving subjects with type 2 diabetes mellitus or metabolic syndrome, we were even able to identify a protective effect of TRT against MACE [21]. Similar conclusions were reached as a joint consensus between the American Association of Clinical Endocrinologists and the American College of Endocrinology in an official position paper that essentially reports that no compelling evidence indicates that T therapy increases CV risk [47].
References
1. Nieschlag E, Nieschlag S. Testosterone deficiency: a historical perspective. Asian J Androl. 2014; 16:161–168. PMID: 24435052.

2. Corona G, Rastrelli G, Maggi M. The pharmacotherapy of male hypogonadism besides androgens. Expert Opin Pharmacother. 2015; 16:369–387. PMID: 25523084.

3. Corona G, Rastrelli G, Vignozzi L, Maggi M. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2012; 17:239–259. PMID: 22612692.

4. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013; 199:548–551. PMID: 24138381.

5. Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and "age-related hypogonadism": FDA concerns. N Engl J Med. 2015; 373:689–691. PMID: 26287846.
6. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013; 173:1465–1466. PMID: 23939517.

7. Muram D, Zhang X, Cui Z, Matsumoto AM. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015; 12:1886–1894. PMID: 26272690.

8. USA Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use [Internet]. Silver Spring (MD): USA FDA;cited 2015 Mar 3. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm.
9. Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012; 120:920–927. PMID: 22996110.
10. Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011; 165:687–701. PMID: 21852391.

11. Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999; 99:1666–1670. PMID: 10190874.

12. Webb CM, Adamson DL, de Zeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol. 1999; 83:437–439. PMID: 10072236.

13. English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000; 102:1906–1911. PMID: 11034937.
14. Thompson PD, Ahlberg AW, Moyna NM, Duncan B, Ferraro-Borgida M, White CM, et al. Effect of intravenous testosterone on myocardial ischemia in men with coronary artery disease. Am Heart J. 2002; 143:249–256. PMID: 11835027.

15. Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, Jones TH, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004; 90:871–876. PMID: 15253956.

16. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009; 161:443–449. PMID: 19542238.

17. Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012; 5:315–321. PMID: 22511747.
18. Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a metaanalysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005; 60:1451–1457. PMID: 16339333.

19. Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007; 82:29–39. PMID: 17285783.

20. Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010; 95:2560–2575. PMID: 20525906.
21. Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014; 13:1327–1351. PMID: 25139126.

22. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013; 11:108. PMID: 23597181.

23. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363:109–122. PMID: 20592293.

24. Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013; 27:557–579. PMID: 24054931.

25. Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011; 34:528–540. PMID: 20969599.

26. Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014; 16:146–152. PMID: 24369149.

27. Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health. 2013; 31:103–125. PMID: 24044106.

28. Guo W, Bachman E, Li M, Roy CN, Blusztajn J, Wong S, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013; 12:280–291. PMID: 23399021.

29. Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014; 11:1577–1592. PMID: 24697970.

30. Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014; 15:1903–1926. PMID: 25080279.

31. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008; 159:507–514. PMID: 18955511.

32. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536–2559. PMID: 20525905.

33. Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013; 10:245–284. PMID: 22971200.

34. Antithrombotic Trialists' (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009; 373:1849–1860. PMID: 19482214.
35. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012; 97:2050–2058. PMID: 22496507.

36. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013; 169:725–733. PMID: 23999642.

37. Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013; 310:1829–1836. PMID: 24193080.

38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014; 311:961–962. PMID: 24595783.

39. Ho PM, Barón AE, Wierman ME. Deaths and cardiovascular events in men receiving testosterone: reply. JAMA. 2014; 311:964–965. PMID: 24595788.
40. Sharma R, Oni O, Gupta K, Chen G, Sharma M, Dawn B, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015; 36:2706–2715. PMID: 26248567.

41. Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014; 48:1138–1144. PMID: 24989174.

42. Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014; 9:e85805. PMID: 24489673.

43. Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy. 2015; 35:72–78. PMID: 25582846.

44. Corona G, Vignozzi L, Sforza A, Mannucci E, Maggi M. Obesity and late-onset hypogonadism. Mol Cell Endocrinol. 2015; DOI: 10.1016/j.mce.2015.06.031. [Epub].

46. Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev. 2012; 8:131–143. PMID: 22268394.

47. Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR. AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015; 21:1066–1073. PMID: 26355962.

Fig. 1
(A) Standardized Mean (95% Confidence Interval [Ci]) Differences In Hematocrit Levels (%) And (B) Mantel-haenzel (Mh) Odds Ratios (95% Ci) For Pathological Hematocrit Elevation (>52%) At Endpoint Between Subjects Treated With Testosterone Supplementation Or Placebo. These Data Were Obtained From A Previous metaanalysis [21].
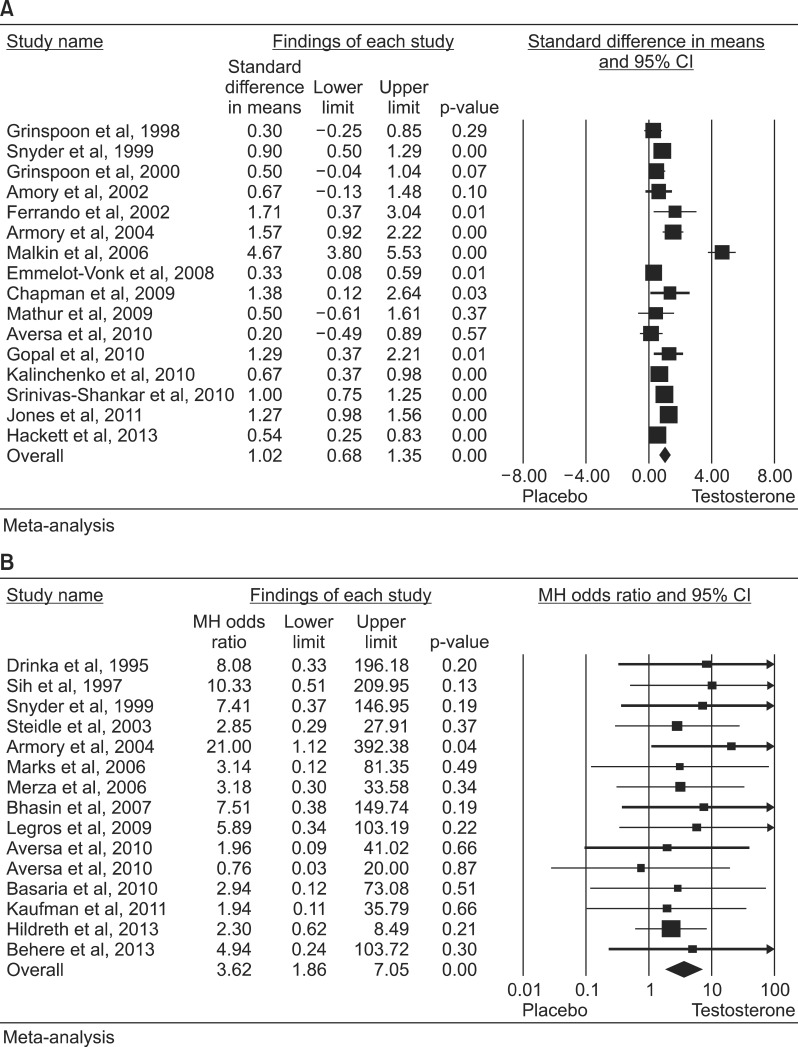
Fig. 2
Odds ratios (95% confidence interval [CI]) for overall mortality (A) and acute myocardial inferction (B) in testosteroneuntreated vs. treated (tosterone replacement therapy) patients. These data were derived from available pharmacoepidemiological studies [35363740414243]. TS: testosterone supplementation.

Table 1
Characteristics and outcomes of the randomized clinical studies included in the meta-analysis
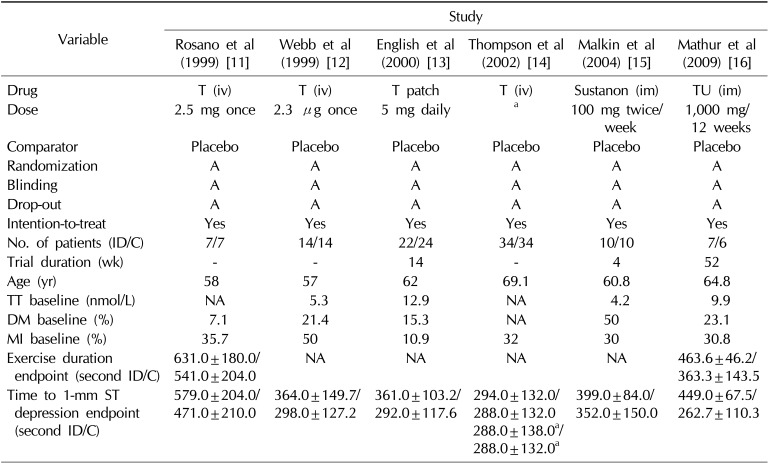
| Variable | Study | |||||
|---|---|---|---|---|---|---|
| Rosano et al (1999) [11] | Webb et al (1999) [12] | English et al (2000) [13] | Thompson et al (2002) [14] | Malkin et al (2004) [15] | Mathur et al (2009) [16] | |
| Drug | T (iv) | T (iv) | T patch | T (iv) | Sustanon (im) | TU (im) |
| Dose | 2.5 mg once | 2.3 µg once | 5 mg daily | a | 100 mg twice/week | 1,000 mg/12 weeks |
| Comparator | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo |
| Randomization | A | A | A | A | A | A |
| Blinding | A | A | A | A | A | A |
| Drop-out | A | A | A | A | A | A |
| Intention-to-treat | Yes | Yes | Yes | Yes | Yes | Yes |
| No. of patients (ID/C) | 7/7 | 14/14 | 22/24 | 34/34 | 10/10 | 7/6 |
| Trial duration (wk) | - | - | 14 | - | 4 | 52 |
| Age (yr) | 58 | 57 | 62 | 69.1 | 60.8 | 64.8 |
| TT baseline (nmol/L) | NA | 5.3 | 12.9 | NA | 4.2 | 9.9 |
| DM baseline (%) | 7.1 | 21.4 | 15.3 | NA | 50 | 23.1 |
| MI baseline (%) | 35.7 | 50 | 10.9 | 32 | 30 | 30.8 |
| Exercise duration endpoint (second ID/C) | 631.0±180.0/541.0±204.0 | NA | NA | NA | NA | 463.6±46.2/363.3±143.5 |
| Time to 1-mm ST depression endpoint (second ID/C) | 579.0±204.0/471.0±210.0 | 364.0±149.7/298.0±127.2 | 361.0±103.2/292.0±117.6 | 294.0±132.0/288.0±132.0 | 399.0±84.0/352.0±150.0 | 449.0±67.5/262.7±110.3 |
| 288.0±138.0a/288.0±132.0a | ||||||
Values are presented as number or mean±standard deviation.
ID/C: investigational drug/comparator, TT: total testosterone, DM: diabetes mellitus, MI: myocardial infarction, T: testosterone, iv: intravenous, im: intramuscular, TU: testosterone undecanoate in castor oil, A: adequate, NA: not available.
aTestosterone doses were individualized to produce physiologic (defined as double the baseline testosterone level) or supra-physiologic (6× baseline) serum testosterone levels.
Table 2
Comparisons of the available meta-analyses evaluating the relationship between TRT and CV outcomes
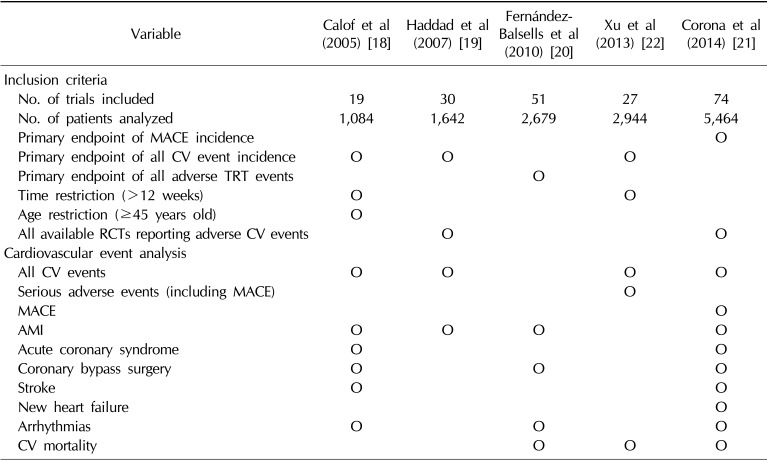
| Variable | Calof et al (2005) [18] | Haddad et al (2007) [19] | Fernández-Balsells et al (2010) [20] | Xu et al (2013) [22] | Corona et al (2014) [21] |
|---|---|---|---|---|---|
| Inclusion criteria | |||||
| No. of trials included | 19 | 30 | 51 | 27 | 74 |
| No. of patients analyzed | 1,084 | 1,642 | 2,679 | 2,944 | 5,464 |
| Primary endpoint of MACE incidence | O | ||||
| Primary endpoint of all CV event incidence | O | O | O | ||
| Primary endpoint of all adverse TRT events | O | ||||
| Time restriction (>12 weeks) | O | O | |||
| Age restriction (≥45 years old) | O | ||||
| All available RCTs reporting adverse CV events | O | O | |||
| Cardiovascular event analysis | |||||
| All CV events | O | O | O | O | |
| Serious adverse events (including MACE) | O | ||||
| MACE | O | ||||
| AMI | O | O | O | O | |
| Acute coronary syndrome | O | O | |||
| Coronary bypass surgery | O | O | O | ||
| Stroke | O | O | |||
| New heart failure | O | ||||
| Arrhythmias | O | O | O | ||
| CV mortality | O | O | O |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download