Abstract
Purpose
Vitis vinifera is a species of Vitis that is native to the Mediterranean region, central Europe, and southwestern Asia, and has been used as a drug in traditional medicine. Traditional medicinal plants have been used for medical purposes with increasing effectiveness. It is important to identify drugs that inhibit spermatogenesis. Therefore, the present study aimed to investigate the effect of grape juice (GJ) on serum levels of inhibin B and sperm count in normal male rats.
Materials and Methods
Thirty-five adult male rats were randomly divided into five groups, each containing seven rats. Rats in the control group received 1 mL of normal saline over the course of the study. The experimental groups received GJ (100, 200, 400, and 1,600 mg/kg, orally, for 35 days consecutively). At the end of the treatment period, fertility indices were measured, including body weight difference, sex organ weight, sperm motility and count, epididymal sperm reserve, daily sperm production (DSP), and serum inhibin B levels.
Results
We found that GJ reduces body weight difference, was associated with decreased sperm motility and count in all treatment groups (p≤0.05 and p≤0.001, respectively). Moreover, DSP was significantly decreased in all treatment groups compared to the control group (p≤0.05), except in the group receiving 100 mg/kg of GJ. Inhibin B levels were significantly decreased in all treatment groups (p≤0.05).
Population control is one of the most important issues in global health, and extensive research in the field of male contraception is being carried out [1]. Some studies on plants that have a history in use in traditional medicine, such as the grape, have suggested that these plants may negatively affect fertility in men. The grape (Vitis vinifera L. Vitaceae) is cultivated worldwide and is of considerable commercial and medicinal importance. In traditional medicine, grapes have been recommended for the treatment of dysentery, pertussis, phthisis, and gastroenteritis. Despite its beneficial effects, its use can be harmful to the kidneys and bladder and may reduce sperm parameters. Iran is one of the most important grape-growing countries, and many Persian cultivars of the grape exist, such as the Asgari and Keshmeshi cultivars [2]. V. vinifera contains many phenolic compounds. Anthocyanins can be found in the skin of grapes, hydroxycinnamic acids in the pulp, and condensed tannins known as proanthocyanidins in the seeds. Stilbenoids can be found in the bark and in the wood. Trans-resveratrol is a phytoalexin produced to inhibit the growth of fungi such as Botrytis cinerea [3].
Grape juice (GJ) contains polyphenols, may increase plasma antioxidant capacity and has protective effects on the vascular system [4]. However, some polyphenols may have harmful effects at high doses or concentrations. In addition, some polyphenols may have antiandrogenic effects, influencing male and female fertility and inducing testicular atrophy [5]. Inhibin is a dimeric glycoprotein that is secreted by the gonads under the stimulation of follicle-stimulating hormone (FSH), and can inhibit FSH secretion and have a corresponding effect on the testis [6]. It is composed of two chains: the α-chain and the β-chain. The β-chain consists of two subunits with different molecular weights. Based on the β-chain, inhibin is divided into two subgroups: A and B [7]. It is well known that inhibin B is the only form of inhibin present in male serum, and is produced by Sertoli cells. Inhibin B can affect the function of the seminiferous epithelium and is regulated by the feedback of the pituitary-gonadal axis. Germ cells and possibly even interstitial cells may produce inhibin as well [8]. Spermatogenesis is dependent on pituitary FSH and on intratesticular testosterone. Previous studies have suggested that inhibin B is a testis-specific marker to check normal gonadal function [9]. In several studies, inhibin B has been found to be a marker on positive spermatogenesis dynamics [10]. Inhibin B levels have likewise been associated with testis volume and sperm count [11]. Therefore, the purpose of the present study was to investigate the effects of V. vinifera (V. Asgari) juice on serum levels of inhibin B and sperm count in normal male rats.
Asgari grapes, which are known to be one of the most important Iranian seedless grape cultivars and are consumed fresh as well as used in raisin production, were purchased from the Center of Fruits and Vegetables in Ahvaz, Iran as V. vinifera. The grapes were washed gently in fresh water to remove any foreign materials. The grapes themselves were removed from the vine, covered with a thin cloth, and were subjected to pressure in order to extract their juice, which was centrifuged and the upper layer was removed. In order to achieve the highest possible concentration, the liquid was placed in an oven at a low temperature. After evaporation of the surface water and drying, the purity of 10% were obtained. The desired doses (100, 200, 400 [12], and 1,600 mg/kg of GJ) were prepared. The concentrated juice was stored at 4℃ until use.
Thirty-five adult male Wistar rats weighing 200±20 g were used in this experiment. The rats were obtained from the animal facility of the Ahvaz Jundishapur University of Medical Sciences (AJUMS). All animals were kept under standard conditions over the course of the experiment, with a reverse 12-hour dark-light cycle (temperature, 20℃±4℃), and were allowed free access to standard diets and water. The experimental protocols were conducted in accordance with the guide for the care and use of laboratory animals issued by the AJUMS and approved by the AJUMS Committee of Ethics in Research. The male rats were randomly divided into five groups of seven animals each; one group was the control, and the other four groups corresponded to different doses of GJ. The rats were allowed to acclimatize in the laboratory for a period of one week before the beginning of the study [13].
Thirty-five adult male rats were used for this study. The rats were divided into five groups as follows. Group 1 was the control group, consisting of five animals who only received 1 mL of physiological saline solution by oral gavage [14]. Four experimental groups consisted of five rats apiece. The animals in these groups received 100, 200, 400, and 1,600 mg/kg of GJ, respectively, by oral gavage. Each rat received a daily dose fed in the late morning, every day for 35 days.
The entire spermatogenic process in rats takes place over 45 days [15], but it has been established that most environmentally induced changes in the sperm occur within 35 days [16]. Therefore, all rats were treated for 35 consecutive days. Before administration, the calculated doses were dissolved separately in distilled water. At the end of the experiment, blood samples were collected for hormonal evaluation. The rats were weighed before and after the experiment.
Twenty-four hours after the end of the thirty-fifth day, the rats were anesthetized using 10% ketamine and 2% xylazine (Alfasan, Woerden, Netherlands), and blood samples were collected from all animals through heart puncture in order to measure inhibin levels. The blood samples from the rats in each group were centrifuged at 3,000 rpm for 15 minutes to separate the sera from the clots and kept in the deep freezer (-20℃) until analysis. Serum inhibin B was measured using a double-antibody solid-phase sandwich enzyme-linked immunosorbent assay (DSL-10-84100i; Diagnostic Systems Laboratories, Webster, TX, USA) using a monoclonal antibody raised against the inhibin-βB subunit, with a detection limit of 7 to 1,000 pg/mL [7]. The weight difference between the first and last day of administration of GJ was recorded. The chest was then opened, and the testis, epididymis, and accessory sex organs (seminal vesicles and prostate glands) were dissected, grossly examined, and weighed. The index weight (IW) of the organ was calculated as: IW=organ weight/body weight×100.
After laparotomy and removal of the testes, the cauda epididymis was removed and placed into 20 mL of fresh physiological saline at the laboratory temperature. Surgical scissors were then used to create a small incision to extract the spermatozoa from the tubules, and the spermatozoa were then homogenized. Next, 10 mL of physiological saline were added to this suspension until an attenuated 10-fold suspension was produced. One drop of the diluted sperm suspension was transferred to each counting chamber of the improved Neubauer (Deep 1/10 mm; LABART, Munich, Germany) and according to established practical methods for sperm counting, white blood cell counts were obtained and the sperm cells were counted under a light microscope at 100× magnification. The testis was placed in 50 mL of fresh physiological saline (37℃) and completely homogenized using a homogenizer. One drop of this material was placed on the Neubauer chamber after two minutes and, as described above, the spermatozoa were counted by an optical microscope at 100× magnification. The number of sperm per testis was calculated using the following formula:
Epididymal sperm reserve=sperm count in 400 small squares×Dilution×1,000 [17]
Cauda epididymal and testicular sperm counts were also carried out and expressed as 106/g of epididymis and 106/mm3, per day respectively, of suspension.
The method by Parandin et al [18] was used for determining the daily sperm production (DSP). In the Wistar rat, developing spermatids spend approximately 6.3 days in spermatogenesis. Thus, to obtain the total DSP and the efficiency (DSP/g) of sperm production, the values obtained above were divided by the number of spermatids per testis and the number of spermatids per grams of testis, respectively.
The data were analyzed using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). The results were expressed as the mean standard error of the means and analyzed for statistical significance using one-way analysis of variance followed by Tukey's test. p values <0.05 were considered to indicate statistical significance.
The effects of GJ on body weight difference and reproductive organ weight are given in Table 1. The oral administration of GJ significantly reduced the body weight difference in comparison with the control group (p<0.001, p<0.05). The results of the post-hoc analysis show an especially significant decrease in body weight difference in the group that received 1,600 mg/kg of GJ compared to the group that received 100 mg/kg of GJ (p<0.001). A significant decline in the weight of the testes and prostate (expressed in mg/100 g of body weight) was observed in the treatment group that received 400 mg/kg of GJ compared with the control group (p<0.05). Moreover, testis weight was significantly increased in the group receiving 200 mg/kg of GJ compared to the groups receiving 100 mg/kg of GJ (p<0.05) and 400 mg/kg of GJ (p<0.001). The weight of the epididymis was significantly lower in the group receiving 100 mg/kg of GJ than in the group receiving 1,600 mg/kg of GJ (p<0.05). The weight of the prostate was significantly greater in the group receiving 100 mg/kg of GJ compared to the group receiving 400 mg/kg of GJ (p<0.05).
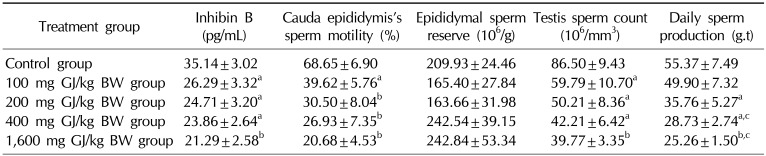
The effect of GJ on inhibin B concentration, sperm motility, sperm count, and DSP are presented in Table 2. Sperm motility, sperm count, and DSP were significantly lower in the treatment groups, especially at higher doses, than in the control group (p<0.05 and p<0.001, respectively). DSP was significantly lower in the groups that received 400 mg/kg of GJ and 1,600 mg/kg of BJ than in the group that received 100 mg/kg of GJ. Inhibin B levels in all treatment groups showed a significant decrease (p<0.05 and p<0.01).
Much research has recently been focused on the anti-fertility effect of plant extracts, especially on the male contraceptive effects of such extracts [1]. These effects may be assessed in a number of ways. Nonetheless, such plants can be considered a convenient and relatively safe method of contraception, especially since the consumption of pharmaceutical contraceptives often causes undesirable side effects. Many antioxidant compounds are found in GJ, which contains flavonoids, catechin, epicatechin, quercetin, and anthocyanins [19].
In this study, we found a decrease in the body weight difference of all experimental groups compared to the control group, and a decrease was also observed in the weight of the reproductive organs (testes and prostate) of the third experimental subgroup (400 mg/kg of GJ) in comparison with the control group. Castilla et al [20] reported that GJ supplementation produced a significant reduction in the concentration of low-density lipoprotein in healthy subjects. On the other hand, Boqué et al [21] have suggested that polyphenolic plant extracts (apple, cinnamon, grape, birch, etc.) may be beneficial for reducing body fat. Our findings are partially in accordance with the above studies. This effect is probably useful to prevent cardiovascular disease. With this in mind, the statistically significant decrease in the weight of the testis and prostate observed in the rats treated with 400 mg/kg of GJ may have been due to decreased testicular and prostate secretions, the reduced volume of their content, or reduced levels of adipose tissue. However, our results did not seem to show dose-dependent outcomes, although the body weight results tended to decrease with increasing doses of GJ. Thus, lower doses are recommended in human use in order to avoid side effects.
This study demonstrated that treatment with GJ significantly reduced sperm motility, count, and DSP in all treatment groups. This reduction was more dramatic at higher doses. Likewise, we found a significant decline in inhibin B levels in all treated groups. The changes in epididymal sperm motility, sperm count, and testicular DSP may have been due to dysfunction in the testis and epididymis, increased apoptosis, decreased gonadotropins, decreased FSH, or decreased testosterone [22]. Inhibin B levels reflect Sertoli cell function and are inversely related to spermatogenesis and age-dependent changes in FSH levels. The Sertoli cells secrete inhibin B into the blood, acting on the pituitary gland to restrict FSH production in response to spermatogenesis. The quantity of inhibin B secreted depends on the activity of the Sertoli cells, meaning that high serum levels of inhibin B indicate good sperm production. Testosterone also affects the brain, leading to restricted FSH production. Moreover, inhibin B has been proposed to be an indicator of testicular function and a possible surrogate for sperm metrics [23]. Illingworth et al [24] reported that inhibin B concentrations were progressively lower in men with increased levels of spermatogenic damage, and are undetectable in men with azoospermia. Possible mechanisms identified for the compounds in the plant extracts in question include the inhibition of gonadotropin secretion [25], interference with steroid genesis at the testicular level [26], and decreasing progesterone concentrations [27]. Another possible mechanism is the inhibition of hyaluronidase activity in rat sperm, which decreases their ability to penetrate the ova, thus interfering with fertility. This effect is probably related to flavonoid activity [28]. Similar research has been done on the reproductive systems of diabetic mice, suggesting that GJ may cause a loss of protein as a result of the unavailability of carbohydrates for producing energy [29]. A study conducted on the effects of celery found that the inhibitory effect of an ethanolic seed extract of Apium graveolens on hyaluronidase activity was associated with its high flavonoid content [22]. Moreover, the findings of this study confirm those of our previous study assessing the effect of a hydroalcoholic extract of V. vinifera leaves on reproductive parameters in adult normal male rats, in which we reported that the administration of this extract affects spermatogenesis and results in the decline of some sperm parameters in the rats [30].
The oral administration of GJ to male rats led to significant reproductive effects. These effects may reflect the reduced levels of inhibin B, which may be responsible for the decline in gonadotropin release, leading to changes in spermatogenesis. It is also possible that the flavonoid content of the extract may have had a direct or indirect effect on the seminiferous tubules, reducing the number of sperm cells. Further experimentation is needed to understand the exact mechanism(s) of the reproductive effects of GJ. The authors of the present study also suggest that the effect of GJ on body weight loss should be studied.
ACKNOWLEDGEMENTS
The Office of Research Affairs of AJUMS financially supported this research (U-89153). The authors would like to express their gratitude for this support. The authors appreciate and thank the Research Deputy Vice-Chancellor for Research Affairs of the AJUMS, and give particular thanks to the Research Consultation Center for technical support.
References
1. Bazrafkan M, Panahi M, Saki G, Ahangarpour A, Zaeimzadeh N. Effect of aqueous extract of ruta graveolens on spermatogenesis of adult rats. Int J Pharmacol. 2010; 6:926–929.

2. Nikkah R, Ebadi A, Naghavi MR, Cresti M, Scali M, Hadadynejad M. Application of SSR markers for characterization of genetic diversity within Iranian grapevine cultivars ('Askari' and 'Keshmeshi'). Hortic Environ Biotechnol. 2010; 51:39–44.
3. Favaron F, Lucchetta M, Odorizzi S, Pais da Cunha AT, Sella L. The role of grape polyphenols on trans-resveratrol activity against Botrytis cinerea and of fungal laccase on the solubility of putative grape PR proteins. J Plant Pathol. 2009; 91:579–588.
4. Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008; 28:729–737. PMID: 19083481.

5. Mennen LI, Walker R, Bennetau-Pelissero C, Scalbert A. Risks and safety of polyphenol consumption. Am J Clin Nutr. 2005; 81:326S–329S. PMID: 15640498.

6. Govand AA, Hamid Ghaffoori H, Aso Omer R. Serum levels of male oligospermia glycoconjugate inhibin B hormone and α-L-fucose in Kurdistani (Iraq) populations. IJBAS-IJENS. 2012; 12:59–66.
7. Dadfar M, Ahangarpour A, Habiby A, Khazaely D. Pre-operative serum level of inhibin B as a predictor of spermatogenesis improvement after varicocelectomy. Urol J. 2010; 7:110–114. PMID: 20535698.
8. Razie S, Panahi M, Ahangarpour A, Rahim F, Saki G. Study of level of inhibin B and ultra structure of sertoli cells in contra-lateral testis after unilateral blunt testis trauma in rat. Asian J Biol Sci. 2011; 4:70–76.

9. Ramaswamy S, Marshall GR, Pohl CR, Friedman RL, Plant TM. Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle-stimulating hormone, respectively, in the rhesus monkey (Macaca mulatta). Endocrinology. 2003; 144:1175–1185. PMID: 12639898.

10. Krause W, Bohring C. Inhibin B as a marker of spermatogenesis. A new dimension in andrology. Hautarzt. 2002; 53:5–10. PMID: 11963224.
11. Buzzard JJ, Loveland KL, O'Bryan MK, O'Connor AE, Bakker M, Hayashi T, et al. Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology. 2004; 145:3532–3541. PMID: 15070852.

12. Hogan S, Zhang L, Li J, Sun S, Canning C, Zhou K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr Metab (Lond). 2010; 7:71. PMID: 20799969.

13. Ahangarpour A, Oroojan AA, Heidari H. Effects of exendin-4 on male reproductive parameters of d-galactose induced aging mouse model. World J Mens Health. 2014; 32:176–183. PMID: 25606567.

14. Kaushik MC, Misro MM, Sehgal N, Nandan D. Effect of chronic oestrogen administration on androgen receptor expression in reproductive organs and pituitary of adult male rat. Andrologia. 2010; 42:193–205. PMID: 20500749.

15. Bullock G, Bunton TE. The laboratory rat. In : Krinke GJ, editor. The handbook of experimental animals: the laboratory rat. New York: Academic Press;2000. p. 150.
16. Gilber SF. Developmental biology. 5th ed. Sunderland, MA: Sinauer Associates;1997. p. 755–758.
17. World Health Organization (WHO). WHO protocol MB-50. A method for the examining the effect of a plant extract administration orally on the fertility of male rats (APF/IP, 9914E). Geneva: WHO;1983.
18. Parandin R, Sadeghipour Rodsari HR, Shamili S, Ghasempour HR. Effects of aqueous extract of Boswellia thurifera on fertility in male rats. ZUMS J. 2009; 16:23–30.
19. Al-Daraji HJ. The use of certain grape constituents for improve semen quality and storage ability of diluted Roosters' semen. Am J Pharm Tech Res. 2012; 2:308–322.
20. Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, et al. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr. 2006; 84:252–262. PMID: 16825703.

21. Boqué N, Campión J, de la Iglesia R, de la Garza AL, Milagro FI, San Román B, et al. Screening of polyphenolic plant extracts for anti-obesity properties in Wistar rats. J Sci Food Agric. 2013; 93:1226–1232. PMID: 23080265.

22. Ola M, Al-Sanabra F, Eyad A, Qunaibi Talal A, Aburjai Farouq A, Al-Qaadan Maha S, et al. Antifertility activity of ethanolic seed extract of celery (Apium graveolens L.) in male albino rats. Jordan J Pharm Sci. 2013; 6:30–39.
23. Anderson RA, Sharpe RM. Regulation of inhibin production in the human male and its clinical applications. Int J Androl. 2000; 23:136–144. PMID: 10844538.

24. Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, Mather JP, et al. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996; 81:1321–1325. PMID: 8636325.

25. Piyachaturawat R, Timinkul A, Chuncharunee A, Suksamrarn A. Growth suppressing effect of curcuma comosa extract on male reproductive organs in immature rats. Pharm Biol. 1998; 36:44–49.

26. Yakubu MT, Akanji MA, Oladiji AT. Evaluation of antiandrogenic potentials of aqueous extract of Chromolaena odoratum (L.) K. R. leaves in male rats. Andrologia. 2007; 39:235–243. PMID: 18076423.

27. Andersen ML, Tufik S. Does male sexual behavior require progesterone? Brain Res Rev. 2006; 51:136–143. PMID: 16386800.

28. Srivastav A, Chandra A, Singh M, Jamal F, Rastogi P, Rajendran SM, et al. Inhibition of hyaluronidase activity of human and rat spermatozoa in vitro and antispermatogenic activity in rats in vivo by Terminalia chebula, a flavonoid rich plant. Reprod Toxicol. 2010; 29:214–224. PMID: 19903524.

29. Ahangarpour A, Oroojan AA, Heidari H, Ehsan G, Rashidi Nooshabadi MR. Effects of hydro-alcoholic extract of Rhus coriaria (sumac) seeds on reproductive complications of nicotinamide-streptozotocin induced type-2 diabetes in male mice. World J Mens Health. 2014; 32:151–158. PMID: 25606564.
30. Afzalzadeh MR, Amirzargar A, Ahangarpour A, Kazemivarnamkhasti M, Ganjali H, Gharibmombeni E, et al. Effects of Vitis vinifera leave hydro-alcoholic extract on reproductive parameters in adult normal male rats. J Phys Pharm Adv. 2013; 6:159–167.
Table 1
Effects of GJ on change in BW and organ weight in adult male rats (n=7)
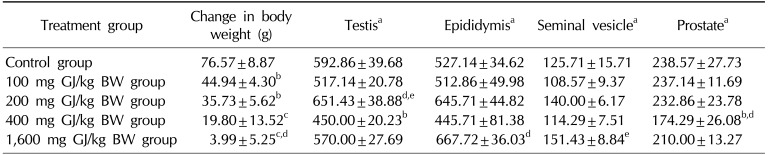
Table 2
Relationship of GJ to sperm characteristics and circulatory levels of inhibin B in adult male rats (n=7)
EDITORIAL COMMENTS
Currently, the use of natural substances, such as plants or herbs, for medical purposes is gaining increased attention in mainstream medicine. The use of these natural substances seems attractive and promising due to their relative convenience and safety, given that chemical drugs often cause undesirable side effects. In practice, many different kinds of natural substances are already used as health supplements or medicine, despite medico-legal issues and a paucity of scientific data.
Animals have been widely used to study the efficacy and safety of natural substances because it is difficult or sometimes impossible to test them in humans. However, the main drawback is that the results from animal studies cannot be directly applied to humans. In order to address this problem, it is essential to use more sophisticated research designs, including both quantitative and qualitative analysis.
In this study, the authors investigated the effect of grape (Vitis vinifera) extracts on the serum level of inhibin B and the sperm count in normal male rats. They concluded that grape extracts could decrease fertility in male rats, although the exact mechanisms were unknown. It is well known that grape extracts are potent antioxidants and free radical scavengers [1], and polyphenolic compounds are responsible for the growing interest in the biologic importance of grape extracts [2345]. The most common polyphenols found in grape extracts are trans-resveratrol, catechin, epicatechin, quercetin and gallic acid [6]. Which of these are associated with fertility? Or are some other constituents of grape extracts involved? The authors suggest that grape extracts could decrease fertility, whereas other studies have shown the protective effects of grape extracts on fertility [7]. The pharmacokinetics and mechanism of action of its isolated compounds may provide an explanation.
In terms of the experimental design of this study, rats received 100, 200, 400, or 1,600 mg/kg of grape extracts. The authors need to explain on what basis the data dosages of grape extracts were determined. Some information on dosage would be useful to contribute to the knowledge on the effects of grape extracts.
In this field, one of the most important factors is the safety of natural substances, which are generally considered safer than chemical drugs. In this study, the body weight of all of the experimental groups significantly decreased compared to that of the control group. These results may mean that grape extracts can cause side effects to some extent.
Despite these shortcomings, the correlation between grape extracts and fertility in this specific study make the study and the manuscript of interest. It is remarkable that grape extracts reduced the sperm motility and counts, and the serum inhibin B level.
The use of natural substances including grape extracts for medical purposes will be inevitable and ongoing, regardless of medico-legal issues and scientific evidence. Therefore, more research should be conducted to establish a concrete scientific basis for whether or not to use grape extracts as natural medicine. Much remains to be learned about grape extracts, and also the values of natural substances in general for medical purposes.
References
1. Chidambara Murthy KN, Singh RP, Jayaprakasha GK. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J Agric Food Chem. 2002; 50:5909–5914. PMID: 12358458.

2. Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997; 11:287–313. PMID: 9292395.

3. Mullen W, Marks SC, Crozier A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J Agric Food Chem. 2007; 55:3148–3157. PMID: 17362029.

4. Favaron F, Lucchetta M, Odorizzi S, Pais da Cunha AT, Sella L. The role of grape polyphenols on trans-resveratrol activity against Botrytis cinerea and of fungal laccase on the solubility of putative grape PR proteins. J Plant Pathol. 2009; 91:579–588.
5. Timperio AM, D'Alessandro A, Fagioni M, Magro P, Zolla L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol Biochem. 2012; 50:65–71. PMID: 21821423.

6. Stagos D, Kazantzoglou G, Theofanidou D, Kakalopoulou G, Magiatis P, Mitaku S, et al. Activity of grape extracts from Greek varieties of Vitis vinifera against mutagenicity induced by bleomycin and hydrogen peroxide in Salmonella typhimurium strain TA102. Mutat Res. 2006; 609:165–175. PMID: 16935024.

7. Sapanidou VG, Margaritis I, Siahos N, Arsenopoulos K, Dragatidou E, Taitzoglou IA, et al. Antioxidant effect of a polyphenol-rich grape pomace extract on motility, viability and lipid peroxidation of thawed bovine spermatozoa. J Biol Res (Thessalon). 2014; 21:19. PMID: 25984501.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download