This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Previous studies have revealed that tamsulosin is effective in improving lower urinary tract symptoms (LUTS) and erectile functioning but has some inhibitory effects on ejaculation, including decreased ejaculatory volume. However, these inhibitory effects on ejaculation can be beneficial to patients with premature ejaculation (PE). Therefore, this study was conducted to understand the effect of tamsulosin on PE in men with benign prostatic hyperplasia.
Materials and Methods
Twenty-nine patients who visited with LUTS were categorized into 2 groups of LUTS-only patients (n=12) and LUTS combined with PE (LUTS+PE) patients (n=17), and 0.4 mg of tamsulosin was administered to the patients of both groups for 12 weeks. Comparative analyses of before and after the treatment were conducted for calculating the International Prostate Symptom Score (IPSS), International Index of Erectile Function-5 (IIEF-5), intravaginal ejaculatory latency time (IELT), premature ejaculation diagnostic tool (PEDT), and premature ejaculation profile (PEP). The patients with an IPSS score of 8 or higher were determined as LUTS patients, and the patients with IELT of less than 2 minutess and a PEDT score of 9 or higher were determined as PE patients.
Results
After treatment, the IPSS score significantly decreased in both groups. There was no statistically significant change in the PEDT for the LUTS group, but there was a significant decrease in PEDT (p=0.012; from 12.1±3.31 to 8.4±4.49) in the LUTS+PE group.
Conclusions
Tamsulosin not only has a treatment effect for LUTS but also improves the PE of LUTS+PE patients. Therefore, further studies are needed to confirm the effects of tamsulosin on PE.
Go to :

Keywords: Ejaculation, Prostatic hyperplasia, Tamsulosin
INTRODUCTION
The morbidity of benign prostatic hyperplasia is extremely high in elderly men. Recently, changes in sexual function before and after the treatment of benign prostatic hyperplasia have attracted attention. Previous studies have reported that tamsulosin, which is used as a primary therapeutic agent for benign prostatic hyperplasia, was effective in the improvement of sexual function as well as the improvement of lower urinary tract symptoms (LUTS) [
1,
2,
3]. However, studies on the effect of tamsulosin on ejaculation have reported that tamsulosin had inhibitory effects in the emission phase of ejaculation, including decreased ejaculatory volume [
4,
5]. On the other hand, the inhibitory effect of tamsulosin on ejaculation could be beneficial to patients with premature ejaculation (PE). In fact, according to a clinical trial study by Akin et al [
6], alpha blocker agents affect the improvement of PE. Although there are numerous definitions of PE, it is likely that the International Society for Sexual Medicine (ISSM)'s recently developed definition will become the
de facto definition. The ISSM proposed that lifelong PE should be defined as male sexual dysfunction characterized by ejaculation that always or nearly always occurs before or within about 1 minute of vaginal penetration; the inability to delay ejaculation on all or nearly all vaginal penetrations; and negative personal consequences, such as distress, botheration, frustration, and/or the avoidance of sexual intimacy [
7]. Thus, in the assessment of the effect of tamsulosin on PE, both intravaginal ejaculatory latency time (IELT) and ejaculatory control ability should be considered. The premature ejaculation diagnostic tool (PEDT) and premature ejaculation profile (PEP), which are questionnaires for following-up the diagnosis or treatment outcomes of PE, have been well validated for their reliability and eligibility in the assessment of ejaculatory control ability, personal distress related to ejaculation, interpersonal difficulty related to ejaculation, and satisfaction with sexual intercourse [
8,
9]. Accordingly, this study was conducted to investigate the effect of tamsulosin on ejaculation in patients with LUTS by comparing the IELT, PEDT, and PEP before and after tamsulosin administration.
Go to :

MATERIALS AND METHODS
This study was a 12-week, single-center, open-label, flexible-dose prospective trial. A total of 43 male LUTS patients were evaluated over 3 months. The appropriate tamsulosin administration dose was determined to be 0.4 mg because some Korean journal reported that the administration of 0.2 mg of tamsulosin had no effect on PE. The inclusion criteria were as follows: men aged 45 to 75 years having a regular sexual partner with active sexual behavior (an IELT score [sexual intercourse ≥2 times/mo], a total International Prostate Symptom Score [IPSS] of ≥8, and a prostate volume of ≥20 mL). The exclusion criteria were as follows: prostate cancer with or without medical or surgical treatment, urethral stricture, erectile dysfunction (International Index of Erectile Function-5 [IIEF-5] score <22), neurogenic abnormality, psychiatric illness, and any kind of cardiovascular abnormality. The participants were evaluated at their first visit to the clinic via a detailed medical history, the recording of vital signs, and a physical examination. Prostate specific antigen, urine analysis, transrectal ultrasonography (TRUS), uroflowmetry (UFM), and residual urine test were also performed for the diagnosis of benign prostatic hyperplasia. In addition, they were asked to complete the IPSS, IIEF-5, PEP, PEDT, and self-reported IELT.
The 43 patients with LUTS were divided into two groups, namely, with and without PE. If a patient had PEDT ≥9 points or self-reported IELT <2 minutes, he was included in the group with PE. The cutoff values of PEDT (9 points) and IELT (2 minutes) in this trial were decided on the basis of the following: Patients with PEDT ≥9 cannot assure not have PE. Therefore, we think that these patients needed to be included in the PE group. Further, the definition of PE in most clinical trials is IELT 2 minutes. Both groups received tamsulosin at a dose of 0.4 mg/d for 12 weeks. The results of the IPSS, UFM, TRUS, IIEF-5, PEP, PEDT, and IELT before and after tamsulosin administration were compared within and between the two groups. IGIC (The clinical global impression of change-scale is a 7-point scale that requires the clinician to assess how much the patient's illness has improved or worsened with respect to a baseline state at the beginning of the intervention and rate it as follows: 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; or 7, very much worse.) results were reported after tamsulosin administration.
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. We carried out the statistical analysis to find the clinical efficacy of tamsulosin on the basis of the IPSS and IIEF-5, and the ejaculatory function by using a paired t-test. A value of p<0.05 was considered statistically significant.
Go to :

RESULTS
1. Demographics
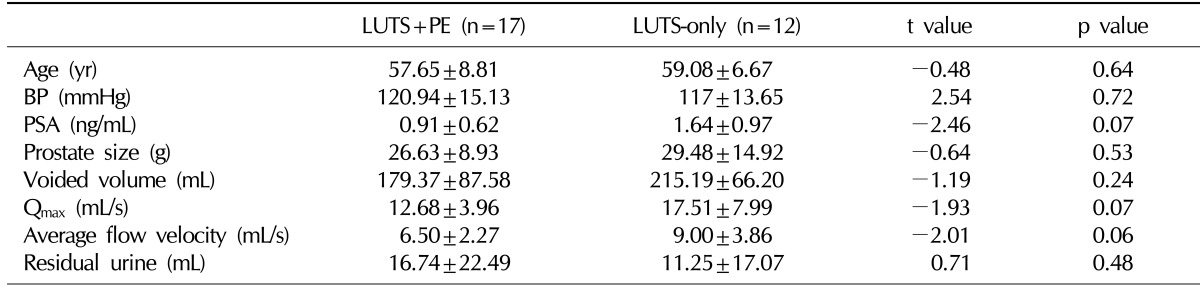
Of the 43 subjects initially included in the study population, 14 were excluded because of incomplete data collection. Eight of the 14 patients maintained the follow-up for 8 weeks, and 6 of the 14 patients maintained the follow-up for 4 weeks because of financial problems or difficulty of accessibility. As a result, 29 men formed the study cohort. The general subject characteristics and baseline data are presented in
Table 1.
Table 1
Pre-treatment characteristics of patients in the LUTS+PE and the LUTS-only groups

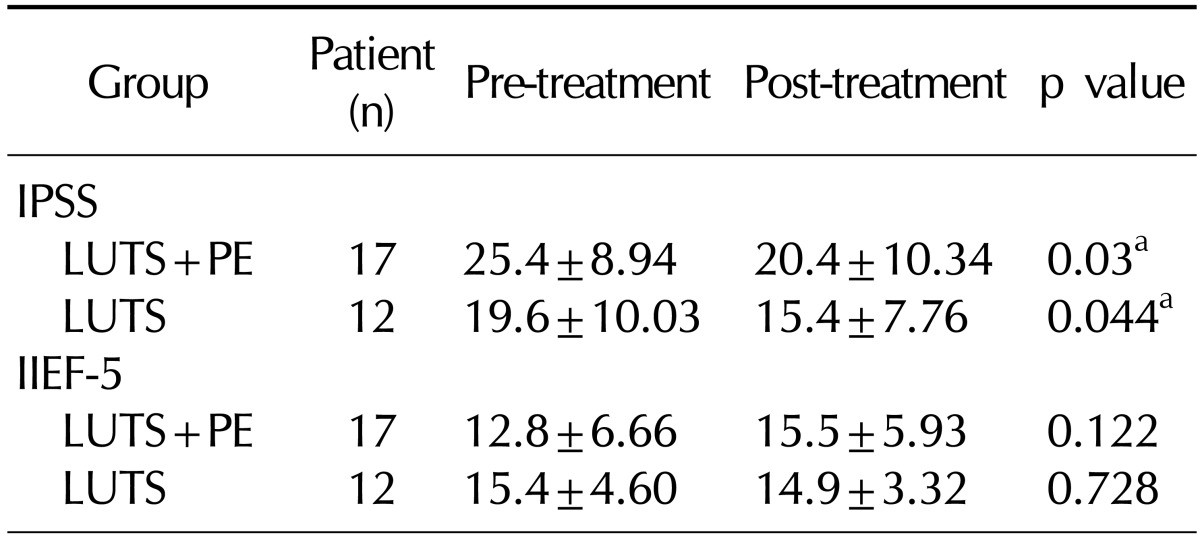
2. Clinical efficacy based on International Prostate Symptom Score and International Index of Erectile Function-5
The IPSS score significantly decreased from 25.4±8.94 before tamsulosin administration to 20.4±10.34 after the 12-week administration of tamsulosin in the LUTS+PE group. Meanwhile, it also significantly decreased from 19.6±10.03 before tamsulosin administration to 15.4±7.76 after the 12-week administration in the LUTS group. However, no significant difference in the IIEF-5 score was found in either group (
Table 2).
Table 2
Effect of tamsulosin on LUTS and erectile function in BPH with or without PE

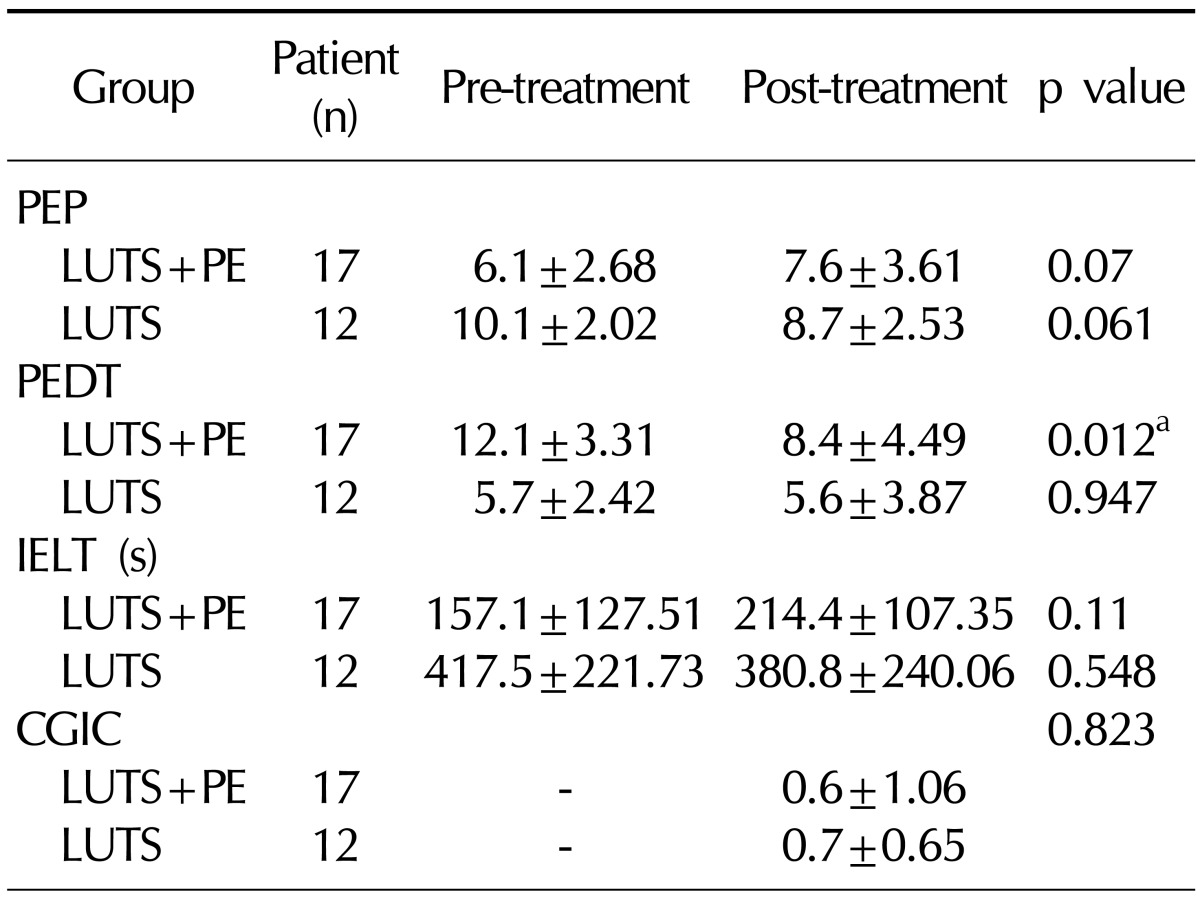
3. Clinical efficacy based on ejaculatory function
The PEDT score significantly decreased from 12.1±3.31 before tamsulosin administration to 8.4±4.49 after the 12-week administration in the LUTS+PE group (p<0.05). Meanwhile, no significant difference was found in the LUTS group. The PEP score increased from 6.1±2.68 before the tamsulosin administration to 7.6±3.61 after the 12-week administration in the LUTS+PE group, but this change was statistically insignificant. No significant difference in the PEP was found in the LUTS group. The IELT increased from 157.1±127.51 seconds before the tamsulosin administration to 214.4±107.35 seconds after the 12-week administration in the LUTS+PE group, but this change was statistically insignificant. The clinical global impression of change in premature ejaculation (CGIC) score increased to 0.6±1.06 and 0.7±0.65 after the 12-week administration in the LUTS+PE and LUTS groups, respectively, but these changes were statistically insignificant (
Table 3).
Table 3
Effect of tamsulosin on premature ejaculation in BPH with or without PE

Go to :

DISCUSSION
Tamsulosin is a typical therapeutic agent for benign prostatic hyperplasia, and its efficacy and safety has been well recognized. In addition, recent studies have reported that the improvement of LUTS by tamsulosin has been helpful in the recovery of sexual function. Meanwhile, with respect to ejaculation, tamsulosin has inhibitory effects such as reduced ejaculatory volume and delayed ejaculation. Therefore, studies have been actively conducted to investigate the causes and the frequency of ejaculatory dysfunction (ED) [
10].
In a phase-III clinical trial conducted by Narayan and Lepor [
11], the rate of ED, erectile dysfunction, and decreased sexual desire was shown to be 30%, 6%, and 5%, respectively, in patients with benign prostatic hyperplasia after administering tamsulosin at a daily dose of 0.4 mg. In a study that extended the above study for 13 weeks or longer, the rate of ED increased from 10% after the administration of tamsulosin at a daily dose of 0.4 mg to 26% after the administration of tamsulosin at a daily dose of 0.8 mg, which showed that the rate of ED increased with an increase in the dose of tamsulosin [
12]. In a study that was conducted by Hellstrom and Sikka [
13], the ejaculatory volume decreased in 90% of the participants and anejaculation was observed in approximately 35% of the participants after the administration of tamsulosin at a dose of 0.8 mg. However, in a study where 0.2 mg of tamsulosin was administered to Korean patients, the rate of ED was significantly lower than that observed in studies performed in other countries. In a study conducted by Lee and Lee [
14], neither erectile dysfunction nor ED occurred after the administration of 0.2 mg of tamsulosin. Cho and Lee [
15] reported that adverse events of decreased sexual desire (8.1%), erectile dysfunction (6.6%), and ED (3.3%) were observed. They interpreted that the decreased rate of ED in Korean patients as compared to foreign studies was attributable to the difference in the tamsulosin dose. On the other hand, another hypothesis was suggested, in which the difference in tamsulosin-induced ED between Korean and Caucasian patients was attributed not to the difference in the drug dose but to the difference in the genetic or ethnic background. Shibata et al [
16] reported that the frequency distribution of a1-adrenoreceptor polymorphism was different between Japanese and American patients, suggesting that the efficacy and adverse events of tamsulosin could vary depending on ethnicity. As a1-adrenoreceptor is distributed in the epididymis,
vas deferens, seminal vesicle, and prostate gland, which are involved in the emission phase of ejaculation, tamsulosin, an a1-adrenoreceptor antagonist, is speculated to have an inhibitory role in the ejaculatory emission phase. Hisasue et al [
17] reported that the decreased contraction of the smooth muscle in the seminal vesicle and
vas deferens made emission difficult, leading to the decreased ejaculatory volume or ED. However, their report was inconsistent with the result of a previous study, showing that tamsulosin-induced ED was attributable to retrograde ejaculation that was caused by the relaxation of the smooth muscle in the prostatic urethra and the bladder outlet. Kim et al [
18] reported that the internal pressure of the seminal vesicle or
vas deferens upon ejaculation decreased in rats compared with the control group after the administration of an a1-adrenoreceptor antagonist. Tamsulosin-induced ED may occur as a negative effect in normal subjects, but it can help ejaculation delay in patients with PE. Accordingly, several studies have been conducted to investigate the effect of tamsulosin on PE. However, few studies have been conducted on the effect of tamsulosin on PE using questionnaires including the IELT, PEDT, or PEP.
In this study, although no significant change in the IELT, PEP, and CGIC was observed, a significant change in the PEDT was observed in the LUTS+PE patients after the tamsulosin administration. The PEDT, a questionnaire that assesses subjective symptoms of PE, consists of 5 questions regarding the ability of ejaculatory control, frequency of PE, severity of PE, severity of stress, and relationship with the sex partner. This result suggests that besides the improvement of LUTS, tamsulosin can improve the subjective symptoms of PE in patients with LUTS+PE. The CGIC and PEP, which assess subjective satisfaction with the treatment of PE, also showed the improvement after the tamsulosin administration, but they were statistically insignificant. This result can be attributed to the small number of patients. The positive effect of tamsulosin on the improvement of PE can be attributed to the increased ejaculatory threshold for sexual stimulation due to the decreased contractility of the seminal vesicle or the
vas deferens by the drug itself. Further, the improvement of PE could be secondarily achieved by the improvement of LUTS. Song et al [
19] reported that the ejaculatory ability increased more in patients who showed more improvement of LUTS after the tamsulosin administration and that the IPSS was closely correlated with the Male Sexual Health Questionnaire Ejaculation Function Domain (MSHQ EjFD) score. Our study also showed that the baseline IPSS score improved in the LUTS+PE patients, and that the IPSS score increased more in the LUTS+PE patients after the tamsulosin administration than in the LUTS-only patients. Thus, a further study is required to see whether the improvement of PE is achieved by the decreased contractility of the seminal vesicle or the
vas deferens by tamsulosin or is secondarily achieved by the improvement of LUTS. Further, the stopwatch IELT more accurately reflects the time of PE than the self-reported IELT. However, our study used the self-reported IELT, and this may have affected the outcome of this study. This is an important limitation of our study design. Thus, a further study using the stopwatch IELT is required.
Go to :

CONCLUSIONS
The PEDT score decreased in the LUTS+PE patients after the daily administration of 0.4 mg of tamsulosin for 12 weeks. The PEP score also improved after the 12 weeks in the LUTS+PE group, but this change had no statistical significance. Further, no significant difference in the IIEF-5 score or the IELT was found in either of the groups. The IPSS score significantly decreased in both the groups. This result suggests the possibility that tamsulosin has a certain positive effect on the improvement of PE in patients with LUTS. A further large-scale study is required to obtain more reliable data in order to assess the effect of tamsulosin on PE.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download