Abstract
Primary seminal vesicle (SV) adenocarcinoma is a rare tumor. A small amount of data about the role of imaging to detect tumor recurrence is available. We report the case of a 58-year-old patient with primary SV clear-cell well-differentiated adenocarcinoma. Clinical and instrumental examinations were negative for the 32 months after treatments when computed tomography scan, [18F]fluoro-D-glucose positron emission tomography/computed tomography and pelvic magnetic resonance imaging showed the appearance of a lesion in the left perineal muscle suspected for recurrence. Patient was symptomless. Cytology of the suspected lesion confirmed SV adenocarcinoma recurrence. The combined approach, using radiological and nuclear medicine techniques, seems to be effective in the follow-up of SV adenocarcinoma. Technological advances, together with awareness of this rare tumor, have the potential of improving patients outcomes not only by providing earlier detection and accurate staging, but also by detecting recurrence and thereby avoiding delays and therapeutic dilemmas.
Primary adenocarcinoma of the seminal vesicle (SV) is very rare and poorly understood neoplasm with approximately 60 documented cases reported in the literature, including adenocarcinoma (the most commonly seen malignant tumor), cystadenoma, and mesenchymal tumor (the most commonly seen benign tumor) [1]. The prognosis is generally poor, and approximately 95% of the patients die in less than 3 years [2].
The symptoms are generally non-specific, including bladder outlet obstruction, hematuria, hematospermia, dysuria, and painful sensation in the pelvis and perineum [3].
Given the rarity of these tumors, there are no established staging or treatment guidelines. The original diagnostic criteria of SV tumors were later modified by Benson et al [2]. Radiological imaging plays a crucial role in the assessment of SV tumors to confirm the presence of the lesion and to evaluate the extent of SV involvement. Usually, they appear as poorly circumscribed solid or cystic masses that may be misinterpreted as an abscess or hemorrhage [1]. Radiology can also help to detect a primary lesion or any associated congenital genitourinary malformations such as ectopic ureters or renal agenesis [3]. 2-deoxy-2-([18]F)fluoro-D-glucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) has been used in the case of SV cancer to stage disease, particularly to exclude distant metastases [3,4] and to assess treatment response [4]. Different from radiological imaging that provides anatomical and morphological data, [18F]FDG-PET/CT characterizes the biological properties of a tumor, depicting lesions with enhanced metabolism. Surgical excision, ranging from local excision or vesiculectomy alone to pelvic exenteration depending on the extent of involvement of the adjacent organs, is the mainstay of treatment. Radiotherapy, multiagent chemotherapy, and androgen deprivation therapy may also be beneficial in the adjuvant or palliative setting [5].
We present the case of a 58-year-old patient with recurrence of SV clear-cell adenocarcinoma associated with right renal agenesis. Since 2006, the patient had suffered from recurrent hematospermia. Serum tumor markers including prostate-specific antigen (PSA), neuron-specific enolase, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA-19.9), alpha-fetoprotein, and chromogranin A were negative. Transrectal ultrasound and pelvic magnetic resonance imaging (MRI) diagnosed bilateral congenital SV cysts. In 2009, a transrectal ultrasound-guided biopsy of the SV was performed due to the persistence of intermittent hematospermia, resulting negative. Since 2010, the patient had also presented episodes of hematuria. No atypical cells were identified in the urinary cytological examination. Cystoscopy was negative; thus, the transrectal ultrasound-guided biopsy of SV was repeated. In the same examination, a bioptical mapping of the prostate was also done. The histology results were consistent with SV adenocarcinoma. Staging examinations included a contrast-enhanced CT scan, [18F]FDG-PET/CT imaging, and bone scintigraphy. In the CT scan, the primary SV lesion was characterized by a hypodense central area presenting peripheral contrast enhancement. Further, a left pararectal lymph node (size: 16 mm) was visualized. [18F]FDG-PET/CT confirmed radiopharmaceutical uptake in the primary SV lesion and in the pararectal lymph node (SUVmax=7.3 and 4.9, respectively). The bone scan was negative. In May 2010, the patient was surgically treated (prostatectomy plus pelvic lymphadenectomy plus bilateral vesiculectomy). Definitive histology confirmed primary bilateral SV clear-cell well-differentiated adenocarcinoma with a tubulo-papillary growth infiltrating the stroma. In the prostate, normal prostatic tissue was not found. The pararectal lymph node resulted metastatic. Immunohistochemistry showed a strong positivity for cytokeratin 7 (CK7), epithelial membrane antigen, and p53, and was negative for PSA, carbohydrate antigen 125 (CA-125), cytokeratin 20 (CK20), caudal-type homeobox protein 2 (CDX2), and CEA.
Thereafter, the patient underwent adjuvant chemotherapy (cisplatinum plus gemcitabine) and intensity-modulated radiation therapy (60 gray). During the first year of follow-up, the patient was followed up by clinical examination, serum tumor markers, and contrast-enhanced CT scan (chest and abdomen) every 3 months. No hematospermia or hematuria occurred. All examinations showed negative results. Thereafter, clinical and radiological examinations were performed every 6 months and all of them showed negative results. In January 2013, a contrast-enhanced CT scan showed the appearance of a lesion (size: 24×15×42 mm) in the left transverse perineal muscle characterized by a hypodense central area with peripheral contrast enhancement (Fig. 1). A thickening of the posterior wall of the bladder was also found. These findings were considered suspicious for tumor recurrence; thus, an [18F]FDG-PET/CT scan was performed. In the PET/CT, the lesion of the left transverse perineal muscle was characterized by a central 'cold' area and peripheral [18F]FDG uptake (SUVmax=4.5), a finding confirmed by the additional acquisition of the pelvis repeated at the end of the whole-body scan, immediately after urination, performed to better visualize the pelvic region (SUVmax=5.8), as shown in Fig. 2. [18F]FDG-PET/CT did not reveal any other pelvic lesions or distant metastasis. A pelvic MRI was also performed. The lesion of the left transverse perineal muscle presented a high signal intensity in T2-weighted images and a low signal intensity in the T1-weighted images, along with wall contrast enhancement after a gadolinium injection, without any cleavage plane with the rectum (Fig. 3). Further, thickening of the posterior wall of the trigone of the bladder was confirmed presenting an internal small hemorrhagic area. The MRI findings were considered suspicious for tumor recurrence although the presence of an abscess could not be ruled out with certainty. The patient did not present any signs or symptoms, and the clinical examination as well as serum tumor markers consistently showed negative results. A transrectal ultrasound-guided cytology of the left transverse perineal muscle lesion was performed in February 2013, confirming the recurrence of SV clear-cell adenocarcinoma. Thus, a chemotherapy regimen with taxol plus carboplatinum (6 cycles) was started, and the patient is currently in follow-up.
We present a case of the usefulness of imaging to detect the recurrence of primary SV clear-cell well-differentiated adenocarcinoma.
Diagnosis of SV adenocarcinoma is difficult and is based on a correlation of clinical, radiological, and histological findings.
Histology is central in this clinical setting, despite the lack of an organ-specific immunophenotype. All reports published about the immunohistochemistry of SV cancers have revealed that these tumors are negative for PSA. The staining for CK7 has usually been strongly positive and that for CK20, negative. The keratin profile might be helpful in distinguishing SV adenocarcinoma from prostate, colorectal, and urothelial-type bladder carcinomas [1,5,6]. In our case, CA-125 was negative, similar to other reports, although cases positive for CA-125 have also been described [5].
Imaging plays a crucial role in identifying primary SV adenocarcinoma. In the imaging, these tumors appear as a mass behind the bladder with or without a prostatic or ureteral obstruction or as an infiltrating lesion in the SV with enhancement similar to that of advanced prostate cancer [7]. Further, imaging can identify renal agenesis or dysgenesis that may be associated with both SV cysts and tumors[8]. Our patient, one year before the diagnosis of SV adenocarcinoma, was diagnosed with a bilateral congenital SV cyst associated with right renal agenesis. SV cysts are commonly associated with renal agenesis or dysgenesis. Incomplete development between the Wolffian duct and the urogenital sinus in males results in an accumulation of secretions and the subsequent formation of SV cysts during puberty [9]. Lee et al [8] suggested that the cause of large SV cysts is not only the ectopic ureter opening into the cyst but also a mucin-producing tumor. On the basis of these premises and our case, we suggest a radiological follow-up of patients with SV cyst, particularly when hematospermia and hematuria are present, in order to eventually detect early SV tumors.
There is little data about the role of imaging in the follow-up of SV adenocarcinoma [4]. In our case, the recurrence of SV adenocarcinoma in the left transverse perineal muscle presented the same characteristic of primary lesion in both contrast-enhanced CT and [18F]FDG-PET/CT; thus, tumor recurrence was suspected. Further, the pelvic MRI findings were considered suspicious although the presence of an abscess could not be ruled out with certainty. Both contrast-enhanced CT scan and pelvic MRI also revealed the thickening of the posterior bladder wall, while [18F]FDG-PET/CT did not show any uptake at this level. Although cytological confirmation of the recurrence of SV adenocarcinoma was obtained only in the left transverse perineal muscle lesion, we cannot exclude the tumor involvement of the posterior bladder wall. In fact, with the excretion of [18F]FDG by the kidney into the urine, intense normal [18F]FDG activity is observed in the intrarenal collection systems, ureters, and bladder, and the urinary excretion of [18F]FDG continued in well-hydrated patients even 1 hour after [18F]FDG administration [10]. Thus, [18F]FDG-PET/CT may give a false negative result in the detection of the recurrence of a bladder tumor. Nevertheless, [18F]FDG-PET/CT allows one to exclude distant metastases.
To summarize, here, we report a case of primary SV clear-cell adenocarcinoma associated with right renal agenesis, recurred after treatments, depicted in both radiology and [18F]FDG-PET/CT. Imaging results were very useful in reaching the final diagnosis. In fact, from a clinical point of view, the patient did not present any suspicious signs or symptoms for the recurrence of SV adenocarcinoma, and both the clinical examination and the tumor markers were yielded negative results. The similarity between the lesion of the left transverse perineal muscle and the primary SV lesion in both the CT scan and [18F]FDG-PET/CT together with the concordance of the findings of both radiological and nuclear medicine techniques led to a strong suspicion of tumor recurrence.
As illustrated by our case, a combined approach using radiology and nuclear medicine techniques seems to be effective in the follow-up of SV adenocarcinoma patients to early diagnose tumor recurrence. Technological advances in imaging and histopathology, together with the awareness of this rare tumor, have the potential of improving the outcomes in these patients not only by providing earlier detection and accurate staging, but also by detecting recurrence and thereby avoiding delays and therapeutic dilemmas.
Figures and Tables
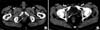 | Fig. 1Computed tomography (CT) images show two lesions suspected for seminal vesicle recurrence. Transaxial CT images show the lesion of the left transverse perineal muscle (arrow in A) associated with a thickening of the posterior wall of the bladder (arrow in B). |
 | Fig. 2[18F]fluoro-D-glucose positron emission tomography/computed tomography ([18F]FDG-PET/CT) images show the lesion suspected for seminal vesicle recurrence. Maximum intensity projection image shows an area of moderate [18F]FDG uptake under the bladder (arrow in A). Transaxial [18F]FDG-PET/CT images show a central 'cold' area and peripheral [18F]FDG uptake at left transverse perineal muscle (arrow in B) as confirmed by images repeated immediately after urination (arrow in C). |
 | Fig. 3Magnetic resonnace imaging (MRI) images show two lesions suspected for seminal vesicle recurrence. Transaxial MRI images show the lesion of the left transverse perineal muscle characterized by a high signal intensity in T2-weighted (arrow in A) and the thickening of the posterior wall of trigone of the bladder in T1-weighted image (arrow in B). |
References
1. Egevad L, Ehrnström R, Håkansson U, Grabe M. Primary seminal vesicle carcinoma detected at transurethral resection of prostate. Urology. 2007; 69:778.

3. Navallas M, Vargas HA, Akin O, Pandit-Taskar N, Fine SW, Eastham JA, et al. Primary seminal vesicle adenocarcinoma. Clin Imaging. 2011; 35:480–482.

4. Kreiner B, Denzinger S, Ganzer R, Fritsche HM, Burger M, Wieland WF, et al. Neuroendocrine carcinoma of the seminal vesicles presenting with Lambert Eaton syndrome: a case report. J Med Case Rep. 2010; 4:320.

5. Oxley JD, Brett MT, Gillatt DA, Burton P. Seminal vesicle carcinoma. Histopathology. 1999; 34:562–563.

6. Tarján M, Ottlecz I, Tot T. Primary adenocarcinoma of the seminal vesicle. Indian J Urol. 2009; 25:143–145.

7. Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP, King BF Jr. Imaging of the seminal vesicle and vas deferens. Radiographics. 2009; 29:1105–1121.

8. Lee BH, Seo JW, Han YH, Kim YH, Cha SJ. Primary mucinous adenocarcinoma of a seminal vesicle cyst associated with ectopic ureter and ipsilateral renal agenesis: a case report. Korean J Radiol. 2007; 8:258–261.

9. Zaontz MR, Kass EJ. Ectopic ureter opening into seminal vesicle cyst associated with ipsilateral renal agenesis. Urology. 1987; 29:523–525.

10. Versari A. Radionuclide imaging of infection and inflammation: a pictorial case-based atlas. In : Lazzeri E, Signore A, Erba PA, Prandini N, Versari A, D'Errico G, editors. Normal findings from different radiopharmaceuticals and techniques, with variants and pitfalls. 1st ed. New York: Springer Science Business Media, Inc.;2013. p. 1–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download