Abstract
Purpose
In the present study, we aimed to identify the incidence of fever in patients after subinguinal microsurgical varicocelectomy and to evaluate the clinical factors associated with the occurrence of the fever.
Materials and Methods
We retrospectively reviewed the cases of patients who underwent subinguinal microsurgical varicocelectomy (group A) under spinal anesthesia. In addition, we reviewed the cases of patients who underwent microsurgical vasovasostomy under spinal anesthesia as a control group (group B). The incidence of fever in each group was compared. We investigated the clinical factors influencing the occurrence of fever in the patients of group A.
Results
The incidence of fever in group A was significantly higher than that in group B (32.5% [53/163] vs. 0.4% [1/284]; p<0.001). Clinical factors such as age, varicocele grade, weight, height, operation time, number of ligated veins, usage of immediate postoperative analgesics, presence of postoperative hematoma, and duration of hospital stay were not significantly associated with the occurrence of fever.
The prevalence of varicocele is estimated to be approximately 15% in the general population [1,2]. Varicocele has a harmful effect on spermatogenesis [3,4]. Although the influence of varicocele treatment on fertility remains controversial, there is clear evidence that varicocelectomy improves the semen parameters that serve as surrogate markers for potential fertility [5,6]. Microsurgical varicocelectomy has been shown to be the most effective method among the surgical techniques available to treat varicocele and produces the least morbidity in patients [7]. Since 1994, subinguinal microsurgical varicocelectomy has become the standard method of varicocele treatment in our medical center.
In our clinical experience, we have noted cases of transient fever following microsurgical varicocelectomy, even in cases where surgery was not performed under general anesthesia. To the best of our knowledge, this issue has not been investigated. In the present study, we aimed to identify the incidence of fever in patients receiving subinguinal microsurgical varicocelectomy and to evaluate the clinical factors associated with the occurrence of the fever.
We retrospectively analyzed the cases of patients with varicocele who were treated by subinguinal microsurgical varicocelectomy (group A) from January 2006 to June 2012 by an experienced surgeon (SW Kim). Cases with prior varicocelectomy or inguinal surgery, performed under general anesthesia; bilateral varicocelectomy; or varicocelectomy performed along with other procedures such as testis biopsy were excluded from the study. The cases of patients receiving microsurgical vasovasostomy under spinal anesthesia were reviewed as the control group (group B). The incidence of fever in each group was compared.
The definition of fever in the present study was an axillary temperature of ≥38.0℃. The duration of fever was defined as the interval between the time at which the fever was first detected and that at which the body temperature had normalized. The relation between the clinical factors and the occurrence of fever was evaluated in group A. These clinical factors included age, grade of varicocele, weight, height, body mass index, anesthesia time, operation time, number of veins ligated, presence of intraoperative arterial injuries, use of immediate postoperative analgesics, presence of postoperative hematoma, and duration of hospital stay (days).
A statistical analysis was performed using the PASW Statistics version 18.0 for Windows (IBM Co., Armonk, NY, USA). The chi-square or Fisher's exact tests were used for the analysis of discrete variables and the independent Student's t-test was used for the analysis of continuous variables. A p value of <0.05 was considered significant. All continuous variables were presented as the median (interquartile range [IQR]).
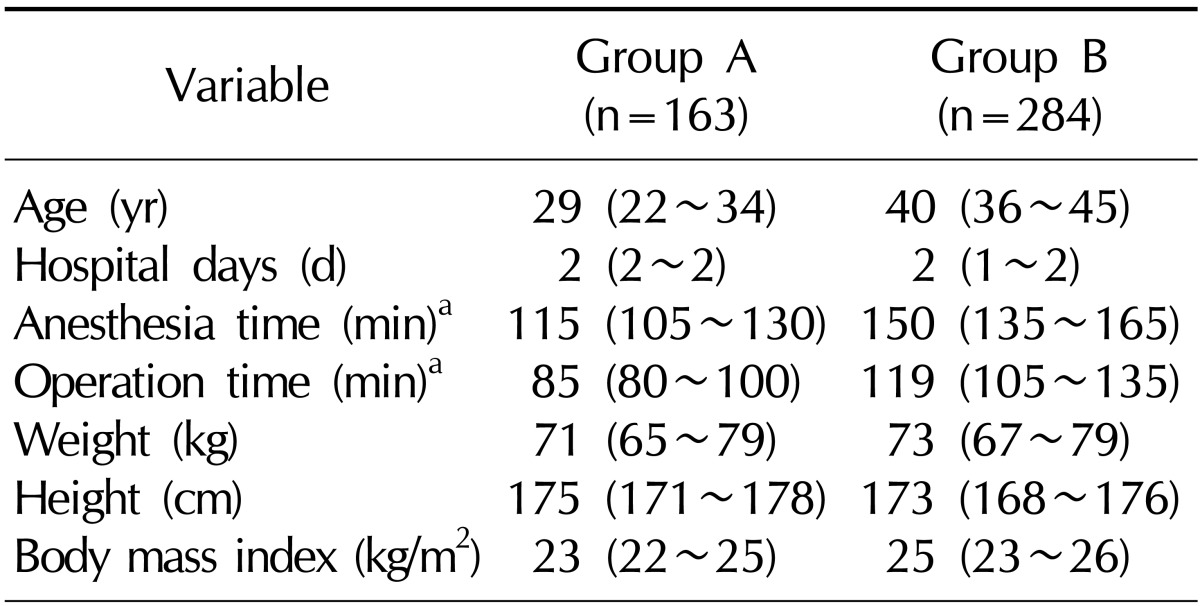
In all, 342 men undergoing subinguinal microsurgical varicocelectomy were identified during the study period. Patients who underwent surgery under general anesthesia (n=137), those who underwent varicocelectomy or inguinal surgery (n=22), those who underwent bilateral varicocelectomy (n=10), and those who underwent varicocelectomy along with other procedures (n=10) were excluded from the study. Thus, 163 cases were eventually included in group A. In this group, all surgeries were performed on the left testis, using spinal anesthesia. Group B-the control group-consisted of 284 cases. The relevant clinical and demographic characteristics of both groups are listed in Table 1. Notably, patients in group A had a significantly greater height (p<0.001) and lower weight (p=0.008) as compared to the patients in group B.
The incidence of fever in group A (32.5%, 53/163) was significantly higher than that in group B (0.4%, 1/284) (p<0.001). When the cut-off value for fever was reduced from 38.0℃ to 37.7℃, a significant difference between the groups with respect to the incidence of fever was still noted (group A: 53.4% [87/163]; group B, 2.1% [6/284]; p<0.001).
In group A, fever occurred at a median of 9.3 hours (IQR: 6.9~13.1 hours) after surgery and persisted for a median of 6.0 hours (IQR: 3.0~12.0 hours). The median peak body temperature was 38.4℃ (IQR: 38.2℃~38.6℃). Fig. 1 illustrates a graph of the vital signs of a patient in group A.
Table 2 shows the data on the relationship between the clinical factors and the occurrence of fever. In the univariate analysis, patients with fever had a higher number of ligated cremasteric veins than those who did not have fever (mean value: 2.50 vs. 2.17; p=0.018). All 5 patients with a scrotal hematoma developed fever postoperatively. In the multivariate analysis, we did not identify any clinical factors that were significantly related to the occurrence of fever.
To the best of our knowledge, this is the first study to report the incidence of fever following microsurgical varicocelectomy. We noted that one-third of the patients in the present study developed transient fever following surgery. To exclude the possible effect of postoperative atelectasis, cases where surgery was performed under general anesthesia were excluded from the study. Cases of patients receiving microsurgical vasovasostomy were used as the control group. This enabled us to confirm that this postoperative fever was not a characteristic of microsurgical scrotal surgery but was developed due to the specific nature of subinguinal microsurgical varicocelectomy.
In other hospitals, most cases of varicocelectomy appear to be performed under general anesthesia. In a recent review article, only 1 of 33 studies used spinal anesthesia as the main anesthetic method for varicocelectomy [8]. Therefore, fever after varicocelectomy may be considered to be related to atelectasis due to the use of general anesthesia.
None of the clinical factors were found to be significantly associated with the development of fever in the present study. A possible cause of fever in these patients could be a transient malfunction in the pampiniform plexus. The spermatic arteries are surrounded by the pampiniform plexus, and therefore, heat exchange can occur between them. However, after varicocelectomy, this heat exchange is abruptly blocked. Moreover, considering that fever involves the entire body and is not a local symptom, pyrogenic cytokines such as tumor necrosis factor, interleukin (IL)-1, and IL-6 may also play a role in the development of fever in these patients. These cytokines induce prostaglandin synthesis in the central nervous system, which leads to the occurrence of fever [9]. Furthermore, given the attenuated febrile response that occurs after vagotomy, an afferent nerve is believed to be a bridge between peripheral inflammation and fever [10].
After arterial embolization of various organs, the postembolization syndrome may also accompany fever and pain postoperatively. This phenomenon may be related to the occlusion of arteries, but not of veins. In the present study, the cases of intraoperative arterial injury were considerably less frequent than those of postoperative fever, and these two conditions were not significantly related. Therefore, the postembolization syndrome does not appear to be associated with fever following varicocelectomy.
In patients undergoing non-urological surgery, general inflammation is believed to be the cause of postoperative fever. Uçkay et al [11] reported that fever following clean orthopedic surgery was not significantly related to any clinical variable, and concluded that this fever was a consequence of general inflammation. The surgical procedures included in our study were microsurgical techniques, which result in considerably less trauma than orthopedic surgeries. Postoperative fever in group B occurred in only 1 patient, suggesting that general inflammation is unlikely to be the cause of fever following microsurgical varicocelectomy.
According to Harrison's Textbook of Internal Medicine, fever is defined as an A.M. oral temperature of >37.2℃ or a P.M. oral temperature of >37.7℃ [12]. In the present study, the axillary temperature was measured instead of the oral temperature, and fever usually occurred around midnight; therefore, this definition of fever could not be applied. In addition, previous studies in the urologic field have defined fever as a body temperature of ≥38.0℃ [13,14,15], based on which we adopted the reference value of 38.0℃ in the present study. Furthermore, two cut-off values of the body temperature for fever were used- 38.0℃ and 37.7℃-to yield results that are more reliable. When using both cut-off values, we noted a significantly higher prevalence of fever in group A.
It has been proposed that greater height and lower body weight are significantly related to the prevalence of varicocele [16]. In the present study, patients of group A had a significantly greater height and lower body weight than those of group B. These findings are comparable to those of the previous study.
During the review of cases in the patient database, we noted that certain doctors did not effectively manage the fever that developed after subinguinal microsurgical varicocelectomy. Although these surgeries were performed under spinal anesthesia, patients were occasionally encouraged to breathe deeply, whereas some underwent blood culture tests. In the present study, we noted that the fever that developed after these surgeries was a benign condition. Therefore, requesting the patient to breathe deeply and performing additional expensive examinations appear to be unnecessary.
This study has several potential drawbacks. First, the intervals of postoperative vital sign examinations were not controlled. The body temperature was measured every 4 hours as a routine procedure. However, in certain cases, the patient's temperature was measured more frequently or less frequently. Second, the amount of hydration that the patient received was not controlled. Third, the number of ligated vessels did not accurately reflect the amount of venous occlusion, as tiny vessels were sealed through Bovie cauterization. Fourth, the pathophysiology of this fever is unclear, and further studies on this issue are required.
References
1. Meacham RB, Townsend RR, Rademacher D, Drose JA. The incidence of varicoceles in the general population when evaluated by physical examination, gray scale sonography and color Doppler sonography. J Urol. 1994; 151:1535–1538. PMID: 8189565.

2. Lee UH. Statistical observation on the varicocele. Korean J Urol. 1970; 11:213–215.
3. Lipshultz LI, Corriere JN Jr. Progressive testicular atrophy in the varicocele patient. J Urol. 1977; 117:175–176. PMID: 833961.

4. Chehval MJ, Purcell MH. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril. 1992; 57:174–177. PMID: 1730313.

5. Choi WS, Kim TB, Paick JS, Kim SW. Factors related to improvement or normalization of semen parameters after microsurgical subinguinal varicocelectomy. Korean J Urol. 2009; 50:39–45.

6. Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, Salonia A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011; 60:796–808. PMID: 21733620.

7. Ding H, Tian J, Du W, Zhang L, Wang H, Wang Z. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int. 2012; 110:1536–1542. PMID: 22642226.

8. Diegidio P, Jhaveri JK, Ghannam S, Pinkhasov R, Shabsigh R, Fisch H. Review of current varicocelectomy techniques and their outcomes. BJU Int. 2011; 108:1157–1172. PMID: 21435155.

9. Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000; 31(Suppl 5):S178–S184. PMID: 11113021.

10. Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain? News Physiol Sci. 1997; 12:1–9.

11. Uçkay I, Agostinho A, Stern R, Bernard L, Hoffmeyer P, Wyssa B. Occurrence of fever in the first postoperative week does not help to diagnose infection in clean orthopaedic surgery. Int Orthop. 2011; 35:1257–1260. PMID: 20871993.

12. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrison's principles of internal medicine. 16th ed. New York: Mcgraw-Hill;2004. p. 104.
13. Gonen M, Turan H, Ozturk B, Ozkardes H. Factors affecting fever following percutaneous nephrolithotomy: a prospective clinical study. J Endourol. 2008; 22:2135–2138. PMID: 18811569.

14. Cadeddu JA, Chen R, Bishoff J, Micali S, Kumar A, Moore RG, et al. Clinical significance of fever after percutaneous nephrolithotomy. Urology. 1998; 52:48–50. PMID: 9671869.

15. Troxel SA, Low RK. Renal intrapelvic pressure during percutaneous nephrolithotomy and its correlation with the development of postoperative fever. J Urol. 2002; 168:1348–1351. PMID: 12352390.

16. Hassanzadeh K, Yavari-Kia P, Soleymanpour H, Ebrahimpour-Tolouei N, Alikhah H. Effect of body mass index on severity and prevalence of varicocele. Pak J Biol Sci. 2011; 14:869–875. PMID: 22518927.
Fig. 1
A graph of the vital signs of a patient in group A, indicating transient postoperative fever. The blue line indicates the axillary body temperature, and the purple line depicts the heart rate of the patient. Notably, body temperature was measured more frequently after the detection of fever.

Table 2
Analysis of clinical variables and the presence of postoperative fever in group A patients
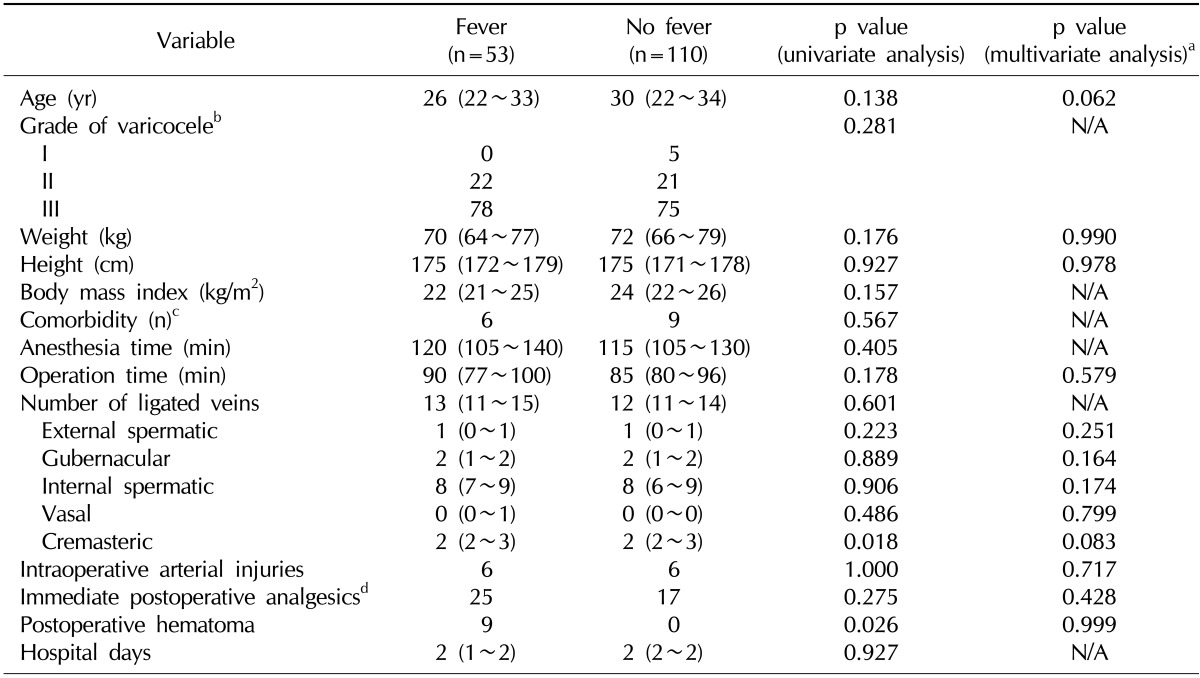
Values are presented as median (interquartile range) or percent.
N/A: not assessed.
aUsing binary logistic regression analysis. bAssessed in 152 cases. cKnown comorbidities at the time of surgery: significant allergy, 4; hypertension, 2; major depressive disorder, 1; autosomal dominant polycystic kidney disease, 1; hyperthyroidism, 1; aortic valve regurgitation, 1; Reye syndrome, 1 (no diabetes mellitus). dAnalgesics administered within 4 hours after surgery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download