Abstract
Purpose
We aimed to evaluate the efficacy of using testicular biopsy histopathology as an indicator of the success of loupe-assisted subinguinal varicocelectomy in non-obstructive azoospermia (NOA) patients.
Materials and Methods
In a 2-year period, a prospective study was carried at Minoufiya University Hospital on 20 NOA patients with clinical bilateral varicoceles. These patients underwent loupe-assisted subinguinal varicocelectomy with simultaneous testicular biopsy. All patients were evaluated by determining their hormonal profile and performing semen analyses and scrotal Doppler and transrectal ultrasonography. Two semen analyses showing azoospermia were performed before the surgery and two semen analyses were received at 3 and 6 months post-operatively for follow-up.
Results
The mean age was 29.9±6.7 years, and the mean follow-up duration was 17.3±8.3 months. We noted the restoration of spermatogenesis in six men (30% of all patients). Testicular biopsy results were as follows: hypospermatogenesis in 7 patients, maturation arrest in 3, and Sertoli cell-only syndrome in 10. The improvement in the sperm counts of these patients ranged from 3 million to 15 million/mL. Sperms were recovered in the hypospermatogenesis (6 patients, 85.5%) patients only, but other patients with testicular biopsy results of Sertoli cell-only or maturation arrest did not show any improvement in their semen parameters.
Varicoceles are the most-frequently diagnosed and correctable cause of male infertility. They are present in 15% of the normal population and 40% of infertile men [1]. They are found in 4.3% to 13.3% of azoospermic patients [2]. Despite extensive research, the exact cause of infertility in men having varicoceles is still unknown, but the benefits of varicocelectomy in terms of sperm concentration, motility, and morphology are well established [3]. Current data in the medical literature on the technique used for varicocelectomy prove that open microsurgical inguinal and subinguinal techniques offer better results than other techniques. However, in terms of achieving a successful spontaneous pregnancy rate, the efficacy of this technique is controversial, and its outcome in azoospermic men remains unclear [4].
Azoospermia is reported in 10% to 15% of infertile men. It is defined as the absence of sperms in an ejaculate in two different semen analyses, and azoospermia management is challenging. Spontaneous pregnancy is almost impossible with azoospermia. New artificial reproductive techniques (ARTs) are considered the only treatment option for non-obstructive azoospermia (NOA) with or without varicocelectomy.
Testicular biopsies can be performed for diagnostic and therapeutic reasons. Testicular spermatozoa can successfully be used for intracytoplasmic sperm injection (ICSI). A diagnostic testicular biopsy may be performed in men with azoospermia, normal testicular volume, and normal reproductive hormones to differentiate between obstructive azoospermia and NOA [5].
The number of reports on male infertility has been continually increasing, particularly of those regarding NOA management. In addition, many studies have discussed the value of varicocelectomy in NOA patients. However, the outcome after varicocelectomy remains controversial, particularly in NOA patients. Moreover, the benefit of performing a testicular biopsy for predicting the outcome of varicocelectomy remains unknown. This study aims to evaluate the effect of loupe-assisted subinguinal varicocelectomy on the improvement of semen parameters in men with NOA by using testicular biopsy histopathology as an indicator.
Between June 2008 and October 2010, a prospective study was carried out at Minoufiya University Hospital, Egypt, on 20 azoospermic patients. All patients had NOA and clinically diagnosed bilateral varicoceles. All of them had primary infertility for at least 1 year. Only cases with clinical varicoceles were selected. Varicoceles were identified by clinical examination and scrotal color Doppler ultrasonography. All patients underwent bilateral loupeassisted subinguinal varicocelectomy. All surgeries were performed by the same team of surgeons, and all pathology examinations were conducted by the same pathologist. Postoperatively, all patients were followed-up regularly in the clinic.
All patients underwent a basic infertility evaluation, including a detailed history. Complete physical examination including a local examination of the testis and scrotum for detecting varicoceles was performed. Scrotal ultrasonography and color Doppler imaging, transrectal ultrasound, hormonal profile, and semen analyses were performed for all patients. Semen samples were collected by masturbation after 2 to 3 days of sexual abstinence. The collected samples were examined in the same laboratory according the World Health Organization (WHO) guidelines [6]. Two semen analyses were performed before the surgery to confirm the azoospermia diagnosis. Postoperatively, two semen analyses were received at 3 and 6 months for follow-up. Patients with a history of undescended testis, testicular trauma, previous urogenital surgery, and genetic sexual problems were excluded from the study.
The technique was performed as described by Esteves and Glina [7]. In brief, a 1-cm skin incision was made over the external inguinal ring. The subcutaneous tissue was dissected until the exposure of the spermatic cord. The cord was elevated with a Babcock clamp. The cremasteric fascia was opened. An optic loupe with ×2.5 magnification was used for all patients. Dilated cremasteric veins within the fascia were ligated and transected. Lymphatics and arteries were identified and preserved. All dilated veins of the spermatic cord were identified, ligated, and transected. Vasal veins were ligated only if their diameters were greater than 2 mm.
All patients underwent bilateral testicular biopsies at the same time as varicocelectomy. In brief, a 1-cm transverse incision was made on the anterior scrotal skin. The tunica was incised until the identification of the white glistening color of tunica albuginea. A 5-mm incision was made over the tunica. A counter pressure was applied on the posterior surface of the testis. The testicular tissue was protruded. A single piece was excised. The specimens were preserved in Bouin's solution. All biopsies were studied histopathologically by an experienced pathologist. According to the histopathology criteria, testicular biopsy specimens were classified as follows: hypospermatogenesis (Fig. 1), maturation arrest (Fig. 2), and Sertoli cell-only (Fig. 3). The biopsy results, postoperative semen analysis results, and the correlation between the induction of spermatogenesis and testicular biopsy were studied.
Calculations were performed using a statistical software package called IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). The significance of the results was estimated by calculating the probability of the chance p value. It was calculated using the chi-square test when comparing between responders and non-responders with respect to histopathology. A Student's t test was used for comparing the parametric numerical data of the responders and the non-responders, and the Mann-Whitney test was used for comparing the non-parametric numeric data of the responders and the non-responders. The Friedman test was used for comparing the sperm counts before surgery with those at 3 and 6 months after surgery.
Twenty patients with bilateral clinical varicoceles and NOA were included in this study. The mean age at surgery was 29.9±6.7 years (range: 19~54 years). The mean follow-up duration was 17.3±8.3 months. All patients underwent bilateral loupe-assisted subinguinal varicocelectomy and testicular biopsy at the same time. No complication resulting from varicocele repair or testicular biopsy was reported postoperatively. The histopathology pictures were identical on both sides. The testicular biopsy results were Sertoli cell-only in 10 cases (50%), early maturation arrest in 3 (15%), and hypospermatogenesis in 7 cases (35%) (Table 1). After varicocelectomy, the semen count improved in 6 hypospermatogenesis cases (6/7; 85.7%), and no improvement was observed at 3 or 6 months in the Sertoli cell-only (germ cell aplasia) and maturation arrest cases (Table 1). In this limited study, significant improvement was noted in the semen parameters of 30% of the total azoospermic patients after varicocelectomy. The mean sperm concentration increased from zero sperm in the ejaculate to 3 million to 15 million/mL after varicocelectomy and after 3 and 6 months post-operation (Table 2). The increase in the sperm counts of the azoospermic patients after loupe-assisted subinguinal varicocelectomy was significant (p=0.013).
We compared the duration of infertility (years), semen volume (mL), follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone, and testicular volume of the responders and the non-responders at 6 months post operation. This comparison showed no significance differences between the responders and the non-responders (Table 3).
The histopathologies of testicular biopsy of the responders and the non-responders were compared. The increase in the sperm counts of the azoospermic patients after varicocelectomy regarding histopathology of testicular biopsy was significant (p=0.001, chi-square: 15.9). Overall, 6 out of 7 (85.7%) of the hypospermatogenesis cases showed an increase in the sperm count to 3 to 15 million sperm/mL. The other 13 cases, with a histopathology of Sertoli cell-only in 10 cases and maturation arrest in 3 cases, showed no increase in their semen count.
Testicular histopathology in severe oligospermic and azoospermic patients ranges from various degrees of hypospermatogenesis to the Sertoli cell-only pattern [8]. Azoospermia accounts for 10% to 20% of males with infertility [9]. Men with NOA can be classified into hypospermatogenesis, maturation arrest, and Sertoli cell-only syndrome according to the cell lining of the seminiferous tubules. Many reports showed incomplete spermatogenesis in the testis of azoospermic patients [10]. Azoospermic men were not able to have children before the advent of ART.
Varicocele repair is the only surgical treatment that has restored sperms to the ejaculate in men with NOA [11,12,13]. In 1952, Tulloch [14] first reported the post-varicocelectomy presence of sperms in the ejaculate of an azoospermic man and spontaneous pregnancy in his partner. Ever since, varicocelectomy has become the most commonly performed surgical treatment for male infertility. A few studies have shown that NOA patients with varicoceles may benefit from varicocelectomy, but the findings remain controversial [12]. Matthews et al [15] studied 22 azoospermic patients and 56 oligoasthenospermic patients. All patients had undergone microsurgical varicocelectomy. Post-operative follow-up semen analyses revealed that 55% of the azoospermic patients and 82% of the oligoasthenospermic patients had motile sperms. The pregnancy rate in the azoospermic group was 14% and that in the oligoasthenospermic group was 38%.
The present study agrees with previous reports indicating that men with NOA can benefit from varicocele repair. Semen parameters were found to be improved in 30% of the NOA patients in our study. A positive effect of varicocelectomy in this patient population has also important implications for ART. No complications resulting from varicocele repair or testicular biopsy, such as bleeding, hydrocele, testicular artery injury, or testicular atrophy, were reported. Serum FSH and testosterone, testis size, and patient age did not statistically affect the outcome. In two different studies, the first study of 28 patients by Kim et al [11] and the second study of 10 patients by Esteves and Glina [7] demonstrated that testicular histopathology was the most important predictive factor of the outcome. They concluded that patients with Sertoli cell-only syndrome and maturation arrest at the spermatocytic stage did not show any improvement in the semen parameters.
In our study, histopathology was the only statistically significant predictor of success among other factors such as duration of infertility (years), semen volume (mL), FSH, LH, testosterone, and testicular volume. Patients with biopsy-proven hypospermatogenesis (85.7%) were the only men with sperm in their ejaculate postoperatively. Overall, this limited study demonstrates that varicocelectomy can be performed safely and effectively in men with NOA because a proportion of such men (30%) continue to produce motile sperm in the ejaculate postoperatively, which can be used for in vitro fertilization (IVF)/ICSI.
Weedin et al [16] reported that testicular histopathology can predict the appearance of sperm in the postoperative ejaculate. Patients with hypospermatogenesis or late maturation arrest have a significantly higher success rate than those with the Sertoli cell-only syndrome or early maturation arrest. Testicular histopathology from a testis biopsy can be used for determining whether patients with NOA will benefit from varicocelectomy. Therefore, it offers patients with NOA the opportunity to have sperms in their ejaculate for performing ICSI and even the possibility of spontaneous pregnancy [2,8,11,17]. Aboulghar et al [18] reported that most NOA men benefitting from varicocelectomy will still require IVF/ICSI to achieve pregnancy. They suggested that the use of fresh, motile, ejaculated sperms in IVF/ICSI provides better results than sperms used from testicular sperm extraction (TESE). Moreover, they considered the use of ejaculated sperms to be technically easier than the use of sperms obtained via TESE.
Several factors should be considered when reviewing previous reports. None of these studies examined the number of the seminiferous tubules in the biopsy. Furthermore, a single-testis biopsy does not provide an accurate image of the entire testis. As such, azoospermic patients who showed the Sertoli cell-only (10 patients in our study) pattern based on single biopsy. In contrast, a large-testis biopsy may show improvements in the semen parameters following varicocelectomy.
Testicular biopsy can be performed easily in the clinic or the operating room with little morbidity and a low complication rate. However, researchers concluded that testicular histopathology based on testicular biopsy could be used for determining whether patients with NOA may benefit from varicocele repair.
Schlegel [19] conducted the cost-effectiveness analysis of varicocelectomy versus ART treatment in infertile couples. Overall, varicocelectomy in infertile men was reported to be a cost-effective alternative to the ART treatment. The mean costs of a live birth following varicocelectomy and ICSI were estimated to be US dollar (USD) 26,268 and USD 89,091, respectively.
The testicular biopsy of NOA patients associated with clinical varicoceles is a simple, quick, and minimally invasive outpatient procedure. Testicular histopathology obtained from a testis biopsy can be used for determining whether patients with NOA will benefit from varicocele repair. Varicocelectomy in patients with NOA can result in the recovery of spermatogenesis and the appearance of motile sperms in the postoperative ejaculate of hypospermatogenesis cases. Patients with hypospermatogenesis have a significantly higher probability of success than those with the Sertoli cell-only syndrome or maturation arrest.
Pasqualotto et al [20] suggested varicocelectomy for all patients with azoospermia and clinical varicoceles. The authors considered the testicular pathology to be focal lesions. Therefore, a single-testis biopsy does not provide an accurate image of testicular pathology, and there is a chance of improvement after varicocelectomy even in the case of germ cell aplasia.
A few studies have mentioned the association between varicoceles and genetic abnormality in infertile men [21,22]. These studies have focused on the outcome of varicocelectomy in infertile men in association with these genetic abnormalities. Kleiman et al [23] reported the significance of the presence of Y microdeletions or karyotype abnormalities in the case of infertility. These abnormalities are observed in 16.6% of azoospermic patients [23]. Therefore, Y chromosome microdeletion patients showed no improvement in their semen parameters after varicocelectomy in NOA, whereas the others showed a few improvements without these genetic abnormalities. Therefore, the results of a chromosomal study of NOA patients may predict the outcome after varicocelectomy. Finally, previous reports and our study recommended that testicular biopsy histopathology and genetic studies are important factors for predicting the successful outcome of varicocelectomy in NOA patients. Further studies with a greater number of patients and a longer follow-up may be needed in the future.
Non-obstructive azoospermic patients may have improved semen quality following bilateral varicocelectomy. This limited number of patients in this study demonstrated that men with azoospermia whose testicular biopsy results indicated hypospermatogenesis have a better chance of improvement in their semen after varicocelectomy in contrast to men having a biopsy result of the Sertoli cell-only syndrome or maturation arrest. Testicular histology could be considered an indicator before proceeding for varicocelectomy repair in men with NOA.
Figures and Tables
Fig. 1
Hypospermatogenesis. Sections show reduced tubular and luminal diameters. There is an overall reduction in germ cell elements. Further, rare mature spermatids are seen. A high-power examination showed the presence of mature spermatids with dark nuclei (H&E, ×200).

Fig. 2
Case of spermatocytic arrest showed a halt of the maturation sequence, usually at the stage of the primary spermatocyte; no spermatids or spermatozoa were present (H&E, ×400).
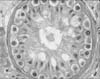
Fig. 3
Tubules are populated by only Sertoli cells with a thickening of the tubular basement membrane; germ cells are completely absent (H&E, ×400).
References
1. Male Infertility Best Practice Policy Committee of the American Urological Association. Practice Committee of the American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril. 2004; 82:Suppl 1. S142–S145.
2. Czaplicki M, Bablok L, Janczewski Z. Varicocelectomy in patients with azoospermia. Arch Androl. 1979; 3:51–55.

3. Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007; 70:532–538.

4. Madgar I, Weissenberg R, Lunenfeld B, Karasik A, Goldwasser B. Controlled trial of high spermatic vein ligation for varicocele in infertile men. Fertil Steril. 1995; 63:120–124.
5. Schlegel PN, Palermo GD, Goldstein M, Menendez S, Zaninovic N, Veeck LL, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997; 49:435–440.

6. World Health Organization. WHO laboratory manual for the examination of human semen and sperm cervical mucus interaction. 3rd ed. Cambridge: The Press Syndicate of the University of Cambridge;1992.
7. Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol. 2005; 31:541–548.

8. Pasqualotto FF, Lucon AM, de Góes PM, Hallak J, Sobreiro B, Pasqualotto EB, et al. Testicular growth, sperm concentration, percent motility, and pregnancy outcome after varicocelectomy based on testicular histology. Fertil Steril. 2005; 83:362–366.

9. Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989; 142:62–65.

10. Silber SJ, Nagy Z, Devroey P, Tournaye H, Van Steirteghem AC. Distribution of spermatogenesis in the testicles of azoospermic men: the presence or absence of spermatids in the testes of men with germinal failure. Hum Reprod. 1997; 12:2422–2428.

11. Kim ED, Leibman BB, Grinblat DM, Lipshultz LI. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999; 162:737–740.

12. Kadioglu A, Tefekli A, Cayan S, Kandirali E, Erdemir F, Tellaloglu S. Microsurgical inguinal varicocele repair in azoospermic men. Urology. 2001; 57:328–333.

13. Cakan M, Altuğ U. Induction of spermatogenesis by inguinal varicocele repair in azoospermic men. Arch Androl. 2004; 50:145–150.
14. Tulloch WS. A consideration of sterility factors in the light of subsequent pregnancies. II. Sub fertility in the male. (Tr. Edinburgh Obst. Soc. Session 104). Edinb Med J. 1951-1952; 59:29–34.
15. Matthews GJ, Matthews ED, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril. 1998; 70:71–75.

16. Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010; 183:2309–2315.

17. Lee J, Binsaleh S, Lo K, Jarvi K. Varicoceles: the diagnostic dilemma. J Androl. 2008; 29:143–146.

18. Aboulghar MA, Mansour RT, Serour GI, Fahmy I, Kamal A, Tawab NA, et al. Fertilization and pregnancy rates after intracytoplasmic sperm injection using ejaculate semen and surgically retrieved sperm. Fertil Steril. 1997; 68:108–111.

19. Schlegel PN. Is assisted reproduction the optimal treatment for varicocele-associated male infertility? A cost-effectiveness analysis. Urology. 1997; 49:83–90.

20. Pasqualotto FF, Lucon AM, Hallak J, Góes PM, Saldanha LB, Arap S. Induction of spermatogenesis in azoospermic men after varicocele repair. Hum Reprod. 2003; 18:108–112.

21. Dada R, Kumar R, Shamsi MB, Sidhu T, Mitra A, Singh S, et al. Azoospermia factor deletions in varicocele cases with severe oligozoospermia. Indian J Med Sci. 2007; 61:505–510.

22. Cayan S, Lee D, Black LD, Reijo Pera RA, Turek PJ. Response to varicocelectomy in oligospermic men with and without defined genetic infertility. Urology. 2001; 57:530–535.

23. Kleiman SE, Yogev L, Gamzu R, Hauser R, Botchan A, Lessing JB, et al. Genetic evaluation of infertile men. Hum Reprod. 1999; 14:33–38.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download