Abstract
Gastrointestinal stromal tumors (GISTs) are an unusual and heterogeneous group of spindle cell tumors that can also appear on the exterior of the gastrointestinal tract (extra-GISTs). Despite the fact that extra-GISTs or large rectal GISTs can lead to the clinical impression of a prostatic mass, these tumors are, in general, excluded in the differential diagnosis of spindle cell tumors observed on prostate needle biopsy. Here, we present, in detail, a case of an extra-GIST identified on prostatic biopsy; the tumor was previously believed to be a primary prostatic stromal sarcoma in the differential diagnosis. Every investigator should check for KIT (CD117) in immunohistochemical staining to rule out an extra-GIST prior to diagnosing a solitary prostatic tumor, specialized prostatic stromal tumor, or leiomyosarcoma on prostate needle biopsy.
Most prostatic spindle cell lesions have very low prevalence and lead to nonspecific laboratory findings and clinical symptoms; they include a widespread display of histopathological entities and are difficult to confirm with a definite diagnosis. Besides, it is more difficult to evaluate these lesions if only a small amount of the specimen is available for prostatic biopsy [1]. Further, gastrointestinal stromal tumors (GISTs) are the most common gastrointestinal mesenchymal tumors and spindle cell neoplasms. The first and second most common sites of these tumors are the stomach and the small intestine, respectively. Only a small percentage of GISTs occur in the rectum. Extra-GISTs are commonly found in the mesentery, greater omentum, and retroperitoneum. Overall, Anagnostou et al [2] reported 20 classified cases of extra-GISTs in the prostate gland, both primary extra-GISTs originating from the prostate and extra-GISTs of the rectum extending to the prostate, by conducting a literature review. We added another seven cases of extra-GISTs presenting as prostatic masses by reviewing the literature published thus far. To the best of our knowledge, this report may be the second case in Korea. Here, we report a case of primary extra-GISTs originating from the prostate and highlight the possibility of extra-GISTs in the differential diagnosis of prostatic spindle cell lesions.
A 50-year-old man visited our hospital with a complaint of weak stream, residual urine sensation, and perineal discomfort. Previously, he had been diagnosed with benign prostatic hyperplasia and had been treated with medication for more than two years at local urologic clinics. Otherwise, he was generally in good health. For these months, he had been experiencing worsening weak stream and urethral pain without any hematochezia or change in bowel habits. Digital rectal examination revealed non-specific findings for the prostate, except that it was apparently enlarged. The serum level of prostate-specific antigen was normal (0.85 ng/mL). Transrectal ultrasonography revealed that a huge prostatic mass measuring 97×88×84 mm was well capsulated with internal hemorrhage and that the mass was isolated from the surrounding structures (Fig. 1A, 1B). The radiologist recommended abdominopelvic computed tomography (CT) and magnetic resonance (MR) imaging for an evaluation of the prostatic mass. CT scan showed direct invasion to adjacent organs with no metastasis. MR imaging of the prostate showed an enlarged prostatic mass with hemorrhagic necrosis. The prostatic mass was large (110×85×86 mm) with heterogeneous enhancement, displacing the bladder anteriorly and rectum posteriorly (Fig. 1C, 1D). This implied that the tumor was mainly localized within the prostate and there was no definite evidence of a direct invasion of adjacent organs. The patient then underwent five-core prostate biopsy guided with transrectal ultrasound. Histologically, the tumor was composed of spindle cells with vesicular nuclei and eosinophilic cytoplasm. These cells were arranged in a whirling or fascicular pattern. There was no significant nuclear pleomorphism (Fig. 2A). Mitotic counts were more than five per 50 high-power fields. The tentative diagnosis was prostatic stromal sarcoma. Tumor cells from the biopsy specimen showed strong and diffuse immunohistochemical reactivity to KIT (CD117) and CD34, while negative immunohistochemical staining results were obtained for desmin, smooth muscle actin, cytokeratin, and S-100 (Fig. 2B, 2C). These outcomes are concordant with the diagnosis of GIST. Therefore, we concluded that the final diagnosis was primary prostatic extra-GISTs. Tumor genotyping was not carried out due to the high costs of this examination. To evaluate the tumor stage, the patient underwent gastroscopy and colonoscopy for the primary lesion, and chest CT and bone scan for the distant metastasis, but there were no abnormal findings in these examinations. We did not start neoadjuvant treatment with imatinib because of its high cost and the patient's lack of medical expense insurance. Therefore, we planned to perform radical prostatectomy routinely; if the tumor had involved the rectum, we would have additionally performed colostomy after complete tumor resection. The patient changed his mind a day before the operation; he refused to undergo radical surgery and left the hospital against our advice. Therefore, we have not followed-up on him since then.
For a long time, the origin and nomenclature of GIST was a subject of much controversy. The putative cell of origin of GIST is the interstitial cell of Cajal (ICC), which is the pacemaker cell of the gastrointestinal muscles. It is known that ICC expresses the gene product of KIT (CD117), a proto-oncogene that encodes the receptor tyrosine kinase, Kit. Because these ICC cells may exist in diverse anatomic sites in addition to the exterior tubular gastrointestinal tract, it is possible to explain unusual cases of extra-GISTs such as those of the uterus, vagina, prostate, and bladder [3].
As the origin of these extra-GISTs is not yet clear, it is difficult to decide whether these tumors are real primary extra-GISTs or secondary sites of GISTs, perhaps apart from the gastrointestinal tract and parasitic growth on another location [2]. The morphologic features of GISTs are variable, and their biological behavior is also difficult to predict. GISTs of the rectum represent only a small percentage (5%) of all GISTs and are often found in males older than 50 years of age [2]. Large rectal GISTs involving the prostate or extra-GISTs originating from the prostate may be interpreted as primary prostatic tumors by using radiological studies and clinical manifestations such as lower urinary tract symptoms, including weak stream, intermittency, and residual urine sensation. Prostate enlargement as a result of extra-GISTs is very unusual. We identified 27 published cases of extra-GISTs diagnosed using prostatic tissue specimens from a review of the literature published to date, including papers on pelvic exenteration, radical prostatectomy, local excision of tumor, and ultrasound-guided prostatic biopsy (Table 1) [3,4,5,6,7,8]. We analyzed the 20 classified cases reported by Anagnostou et al [2] and found the other seven cases ourselves. The median age of all patients was 56.8 years (age range: 42~82 years), and the clinical symptoms were mainly lower urinary tract symptoms (voiding difficulty, hematuria, and/or dysuria) and/or lower gastrointestinal tract symptoms (perianal discomfort and/or small stool diameter). The tumor size of extra-GISTs presenting as prostatic mass varied considerably (range: 1~15 cm); most of these tumors were very huge, occupying a large volume of the pelvic area. This was observed in our case as well (11 cm). Tumors from the 20 cases identified by Anagnostou et al [2] involved both the prostate and the rectum; those from the two other cases were believed to originate from the space between these two organs. These cases may demonstrate extension into the prostate from a primary rectal origin. A small percentage, five cases (18.5%), of the published reports reported a primary prostatic origin [4,5,6,7,8,9]. Further, only three of these cases were confirmed to originate not from rectal GISTs but from primary prostatic GISTs through complete specimens obtained by radical surgery [5,8,9]. In the case reported by Arce-Lara et al [6], the researchers were not confident of their decision regarding the origin of the tumors and therefore, were unable to regard the tumors as primary prostatic extra-GISTs due to the proximity of the rectum and the patient's response to the neoadjuvant treatment with a tyrosine kinase inhibitor such as imatinib.
Further, although the present report may be presumed to be the second case of primary prostatic extra-GIST in Korea, there are limitations to this report. A weakness in the present report is that because the patient did not undergo radical surgery, our case was based only on prostatic biopsy and radiological studies, like the case reported by Van der Aa et al [4]. On the basis of the same principle, it is not certain whether this tumor was really a primary prostatic extra-GIST.
On the contrary, because neoadjuvant therapy with a tyrosine kinase inhibitor such as imatinib has a large effect on the patient's prognosis, it is very important to distinguish between extra-GISTs presenting as prostatic masses and other primary prostatic tumors of spindle cell lesions. Further, immunohistochemical outcomes are the most useful methods for distinguishing between them. Primary prostatic extra-GISTs presented uniformly strong positive responses for both KIT and CD34 stains, while the other prostatic tumors only presented positive responses for CD34 stain only [5].
Further, Fletcher et al [10] proposed the consensus criteria for defining the risk of aggressive behavior in GISTs at the National Institutes of Health. He created a classification of risk groups (low, intermediate, and high) of GISTs using tumor size and mitotic count as predictable values. According to these criteria, the patient fell into the high-risk category and should have been promptly treated with imatinib and radical surgery.
To summarize, we report an unusual case of extra-GIST originating from the prostate as the primary site. It is important that clinicians take great caution with the diagnosis of all patients, such that rectal invasion first be excluded in order to confirm the diagnosis of primary prostatic extra-GISTs.
We concluded that extra-GISTs should always be included in the differential diagnosis of any prostatic spindle cell tumor. Early testing by means of positive KIT (CD117) results will lead to suggestions of immediate imatinib neoadjuvant therapy or surgical resection.
Figures and Tables
Fig. 1
(A, B) Transrectal ultrasonography revealed that the huge prostatic mass measuring 97×88×84 mm was well capsulated with internal hemorrhage. The mass was isolated from the surrounding structures. (C, D) Magnetic resonance image of the prostate showed an enlarged prostatic mass with hemorrhagic necrosis. The prostatic mass had a large size (110×85×86 mm) with heterogeneous enhancement and displaced bladder (arrow) anteriorly and rectum (arrowhead) posteriorly. This implies that the tumor was mainly localized within the prostate and there was no definite evidence of the direct invasion of adjacent organs.
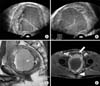
Fig. 2
(A) The tumor is composed of relatively uniform spindle cells with vesicular nuclei and eosinophilic cytoplasm. These cells are arranged in whorled or fascicular pattern. There is no significant nuclear pleomorphism (H&E, ×200). (B) CD117 (c-kit) staining shows diffuse strong positive immunoreactivity in the cytoplasm of the neoplastic cells (×200). (C) Tumor cells also demonstrate diffuse strong cytoplasmic positive inmmunostaining for CD34 (×200).

ACKNOWLEDGEMENTS
This work was supported by a research grant from Jeju National University Hospital.
References
1. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007; 20:148–158.

2. Anagnostou E, Miliaras D, Panagiotakopoulos V. Diagnosis of gastrointestinal stromal tumor (GIST) on transurethral resection of the prostate: a case report and review of the literature. Int J Surg Pathol. 2011; 19:632–636.
3. Herawi M, Montgomery EA, Epstein JI. Gastrointestinal stromal tumors (GISTs) on prostate needle biopsy: a clinicopathologic study of 8 cases. Am J Surg Pathol. 2006; 30:1389–1395.
4. Van der Aa F, Sciot R, Blyweert W, Ost D, Van Poppel H, Van Oosterom A, et al. Gastrointestinal stromal tumor of the prostate. Urology. 2005; 65:388.

5. Yinghao S, Bo Y, Xiaofeng G. Extragastrointestinal stromal tumor possibly originating from the prostate. Int J Urol. 2007; 14:869–871.

6. Arce-Lara C, Shah MH, Jimenez RE, Patel VR, Benson DM Jr, Clinton SK, et al. Gastrointestinal stromal tumors involving the prostate: presentation, course, and therapeutic approach. Urology. 2007; 69:1209.e5–1209.e7.
7. de la Roza G, Naqvi A, Clark K. Gastrointestinal stromal tumors presenting as a prostatic mass. Can J Urol. 2009; 16:4502–4506.
8. Park SW, Lee W, Huh GY, Chung MK. Gastrointestinal stromal tumor of prostate. Korean J Urol. 2008; 49:383–385.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download