Abstract
Purpose
Experimental studies have suggested that the stromal-derived factor-1 (SDF-1)/CXCR4 axis is associated with tumor aggressiveness and metastasis in several malignancies. We performed a meta-analysis to elucidate the relationship between CXCR4 expression and the clinicopathological features of prostate cancer.
Materials and Methods
Data were collected from studies comparing Gleason score, T stage, and the presence of metastasis with CXCR4 levels in human prostate cancer samples. The studies were pooled, and the odds ratio (OR) of CXCR4 expression for clinical and pathological variables was calculated.
Results
Five articles were eligible for the current meta-analysis. We found no relationship between CXCR4 expression and Gleason score (<7 vs. ≥7). The forest plot using the fixed-effects model indicated an OR of 1.585 (95% confidence interval [CI]: 0.793~3.171; p=0.193). Further, CXCR4 expression was not associated with the T stage (<T3 vs. ≥T3), and the relevant meta-analysis showed OR=1.803 (95% CI: 0.756~4.297, p=0.183). However, increased CXCR4 expression was strongly associated with metastatic disease with a fixed-effects pooled OR of 7.459 (95% CI: 2.665~20.878, p<0.001).
Prostate cancer is the most commonly diagnosed male malignancy and is the second leading cause of cancer deaths for men in the Western world [1]. Radical surgery or radiotherapy can be curative therapy for patients with localized prostate cancer. However, approximately 15% to 20% of men with prostate cancer eventually experience metastatic disease, and androgen deprivation treatment is the most effective systemic approach for patients with metastatic disease. Although 80% to 90% of patients initially respond favorably to this treatment, they eventually become unresponsive to androgen deprivation and develop castration-resistant prostate cancer (CRPC) and are subsequently at risk of death [2,3]. Serum prostate-specific antigen (PSA) measurements have been used for early detection of prostate cancer, prediction of tumor aggressiveness, prognosis, selection of treatment modality, and monitoring of treatment outcomes. Absolute PSA levels and other measures of PSA kinetics can be useful in predicting bone metastasis, but some limitations remain for the application of PSA parameters in various clinical settings. Accordingly, much research has been focused on discovering other novel biomarkers that predict the development of metastases more accurately [4].
Stromal derived factor-1 (SDF-1) is a member of the CXC subfamily of chemokines that interact with the seven-transmembrane G-protein-coupled receptor CXCR4 [5]. CXCR4 expression has been reported in at least 23 epithelial, mesenchymal, and hematopoietic cancers, suggesting the importance of this ligand/receptor axis in tumor aggressiveness and metastasis [6]. In addition, the role of the SDF-1/CXCR4 axis in prostate cancer has been experimentally demonstrated. SDF-1 binding to CXCR4 generates various signaling mechanisms that affect the regulation of angiogenesis, activation of cell invasion, promotion of cell growth, and inhibition of apoptosis, and notably, plays an important role in organ-specific metastasis [7,8,9,10,11]. Several researchers have demonstrated in human sample studies that increased CXCR4 expression in prostate cancer is associated with tumor aggressiveness, metastatic disease, and poor survival outcome [12,13,14,15,16,17,18]. However, their results were somewhat contradictory and inconclusive because the number of tested samples in each study was relatively small. Herein, we performed a meta-analysis to elucidate the relationship between CXCR4 expression and the clinicopathological features of prostate cancer.
This meta-analysis was designed and conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (accessible at http://www.prisma-statement.org/) [19]. Eligible studies were identified after electronic searching of databases. A comprehensive search of the PubMed and EMBASE databases was performed using keywords and the medical subheadings of 'CXCR4' and 'prostate'. Alternative spellings or abbreviations of these keywords were also used. There were no research limitations, and the most recent study was performed on September 3, 2013.
Two investigators (J.Y.L. and D.H.K.) independently selected eligible trials. Studies met the following criteria: (1) case-control or cohort studies, (2) immunohistochemical studies with human prostate samples to investigate the association between CXCR4 and the clinicopathological features of prostate cancer including Gleason score, T stage, and the existence of metastasis, and (3) published full-text articles. Studies without detailed patient data were excluded. Disagreement between the two investigators was solved by discussion with another investigator (K.S.C.).
One researcher (J.Y.L.) screened the titles and abstracts identified by the search strategy. The other two researchers (D.H.K. and H.L.) independently evaluated the full text of the papers to determine whether they met the inclusion criteria. The databases were designed to ensure that the most relevant data were obtained with respect to author, publication year, CXCR4 expression, T stage, Gleason score, and the presence of metastatic disease. Disagreements were resolved by discussion until a consensus was reached or by arbitration employing another researcher (K.S.C.).
Upon selecting the final group of articles, two researchers (D.H.K. and J.K.K.) independently examined the quality of each article by using the Scottish Intercollegiate Guidelines Network (SIGN), which is a quality assessment tool for observational studies [20]. This system is internationally accepted and used by guideline developers. Similar rating scales have been published by the Society for Prevention Research [21] and Kumpfer and Alvarado [22]; however, these require higher levels of evidence (when such evidence comes from randomized controlled trials or case-control trials performed by multiple independent research groups) and stricter criteria for assessing the quality of the research [23]. For quality assessment, the design quality of a study was categorized as follows: 'low' (score 0~14); 'modest' (score 14.5~19); 'good' (score 19.5~24); or 'very good' (score 24.5~30).
Heterogeneity among the studies was explored using the Q-statistic and Higgins' I2 statistic [24]. Higgins' I2 statistic measures the percentage of total variation due to heterogeneity rather than chance across studies. Higgins' I2 is calculated as follows: where 'Q' denotes Cochran's heterogeneity statistic and 'df' indicates the degrees of freedom.
An I2 value greater than 50% represents substantial heterogeneity. For the Q-statistic, heterogeneity was deemed significant if p<0.10 [25]. When there was evidence of heterogeneity, data were analyzed using a random-effects model to obtain a summary estimate for the test sensitivity with 95% confidence intervals (CIs). In studies in which positive results were confirmed, a pooled specificity was calculated with 95% CIs.
When Q-test values indicated heterogeneity across studies (p<0.10 or I2>50%), the random-effects model was used for the meta-analysis. Otherwise, the fixed-effects model was employed [26]. Begg and Mazumdar's rank-correlation tests and Egger's regression intercept test were used to examine the evidence of publication bias [27,28], which was depicted as a funnel plot (p<0.05 was considered a significant publication bias). A meta-analysis of comparable data was performed using R (R version 3.0.2; R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org), and its meta and meta- for packages were used for pair-wise meta-analyses.
Searching the databases resulted in 141 articles that could be potentially included in our meta-analysis. On the basis of the abovementioned inclusion and exclusion criteria, 70 articles were excluded after an evaluation of the titles and abstracts. We reviewed the full text of the remaining articles, and 11 articles were selected as potential candidates for our meta-analysis. Six articles, which did not fit the eligibility criteria of this meta-analysis, were excluded. Finally, five articles were included in our analysis of the relationship between CXCR4 and the clinicopathological features of prostate cancer [12,13,14,15,16] (Fig. 1). Data corresponding to confounding factors derived from each study are summarized in Table 1. The results of the final quality assessment showed that of the five articles included, three scored 'low' and two were classified as 'modest' according to the SIGN checklists. The most frequent quality assessment issue was poor selection of subjects.
Heterogeneity was examined using forest plots, as shown in Fig. 2. A heterogeneity test showed the following: χ2=3.99 with 3 df (p=0.262) and I2=24.9% in the analysis of Gleason scores between <7 and ≥7; and χ2=2.05 with 3 df (p=0.562) and I2=0% in the analysis between stage <T3 and stage ≥T3. In the analysis for metastatic prostate cancer, a heterogeneity test also demonstrated homogeneity with χ2=0.19 with 3 df (p=0.86) and I2=0%. Because there were no heterogeneities in three forest plots, fixed-effects models were applied using the Mantel-Haenszel method. The radial plots revealed no heterogeneous variables after the selection of effects models (Fig. 3).
Begg and Mazumdar's rank-correlation tests revealed no evidence of publication bias between Gleason scores of <7 and ≥7 in the present meta-analysis (p=0.333). With respect to T stage and metastasis, a significant publication bias was observed (p=0.083 in two meta-analyses). However, Egger's regression intercept test also revealed no evidence of publication bias in two meta-analyses for T stage (p=0.171) and metastasis (p=0.400). Using the results of these three meta-analyses, we drew the funnel plot shown in Fig. 4.
We observed no relationship in a meta-analysis regarding CXCR4 expression and Gleason score (<7 vs. ≥7). The forest plot using the fixed-effects model demonstrated an odds ratio (OR) of 1.585 (95% CI: 0.793~3.171, p=0.193). Additionally, CXCR4 expression was not associated with T stage (<T3 vs. ≥T3), and the relevant meta-analysis showed an OR of 1.803 (95% CI: 0.756~4.297, p=0.183). However, higher CXCR4 expression was strongly associated with the presence of metastatic disease, with a fixed-effects pooled OR of 7.459 (95% CI: 2.665~20.878, p<0.001).
The data from this meta-analysis indicated that increased CXCR4 protein expression in prostate cancer specimens is significantly associated with the presence of metastatic disease, but not with Gleason scores or T stage. The SDF-1/CXCR4 axis has been experimentally shown to play an important role in organ-specific metastasis of prostate cancer, and several studies with human samples have compared tumor aggressiveness, metastatic disease, and survival outcome with CXCR4 expression levels. However, the numbers of samples tested in each study were too small to achieve adequate statistical power. For example, there were four studies in this meta-analysis evaluating CXCR4 expression and metastasis. However, the study by Mochizuki et al [13] was the only study to demonstrate with statistical significance that higher CXCR expression is associated with metastatic disease. Although the other three studies revealed similar tendencies, their results were statistically not significant, predominantly due to the small sample sizes [12,14,15]. Thus, our meta-analysis provides meaningful clinical and pathological evidence that strongly supports previous experimental data regarding the role of the SDF-1/CXCR4 axis in prostate cancer metastasis. However, our analysis was limited by the small number of included studies.
It is known that the binding of chemokines to their G protein-linked receptors on target cells leads to a series of signal transduction events involving the generation of inositol 1, 4, 5-triphosphate and cyclic adenosine monophosphate-dependent protein kinase, activation of phosphatidylinositol 3-kinase (PI3K), phosphorylation of protein kinase B (Akt), phosphorylation of extracellular signal-regulated kinase (ERK), elevation of components of focal adhesion complexes, and activation of protein kinase C [29]. SDF-1 binding to CXCR4 generates various signaling mechanisms that regulate angiogenesis, activate cell invasion, promote cell growth, inhibit apoptosis, and notably, play an important role in organ-specific metastasis. In a previous study on prostate cancer, differential activation of the ERK and PI3K/Akt pathways resulted in differential secretion of interleukin (IL)-6, IL-8, tissue inhibitors of metalloproteinase-2, and vascular endothelial cell growth factor (VEGF), which affected the ability of the cancer cells to induce angiogenesis [7]. Exogenous SDF-1 induces Akt phosphorylation in PC-3 cells, which is independent of PI3K and indispensable for matrix metalloproteinase (MMP)-9 secretion, migration, and invasion [8]. SDF-1 induction enhances various MMPs in PC-3 cells [9]. It has also been reported that SDF-1-induced expression of CXCR4 in PC-3 cells is dependent on the mitogen-activated protein Kinase Kinase (MEK)/ERK signaling cascade and on nuclear factor kappa B (NF-κB) activation, which enhances endothelial adhesion and transendothelial migration [10]. Additionally, Wang et al [11] showed that CXCR4 plays an important role in prostate cancer metastasis via the up-regulation of VEGF.
Androgen deprivation therapy is effective as an initial strategy in the management of metastatic prostate cancer; however, it generally fails to obtain long-lasting efficacy. Thus, metastatic prostate cancer becomes CRPC, which is no longer responsive to hormonal manipulation. Unfortunately, there are no effective treatment modalities for the management of CRPC. The combination of docetaxel and prednisone has been regarded as standard first-line therapy for CRPC during the past decades, but the survival gain from docetaxel chemotherapy is limited and unsatisfactory [30]. There have been great efforts to discover new molecular targets and develop novel agents based on the advanced understanding of prostate cancer biology. Researchers and physicians have focused on treatment strategies targeting steroidogenesis, androgen receptor, angiogenesis, other growth and survival pathways, and immune response [31]. Recently, novel drugs have been approved for CRPC patients. Sipuleucel-T, cabazitaxel, abiraterone acetate, radium-223, and enzalutamide have shown improved overall survival outcomes in randomized phase III trials; nevertheless, metastatic CRPC still remains incurable [31].
Because metastasis greatly influences the prognosis and treatment of advanced prostate cancer, targeting the SDF-1/CXCR4 axis is a potentially attractive strategy because it emphasizes prevention or delay of metastatic disease. Recent studies have shown promising experimental data, suggesting that CXCR4 antagonism can be an effective modality to control metastatic disease by disrupting the interaction between cancer cells and the protective microenvironment [32,33]. Domanska et al [32] reported that CXCR4 inhibition sensitizes prostate cancer cells to docetaxel in vitro and in vivo. Cho et al [33] found that CXCR4 antagonism significantly inhibited microvessel formation and tumor growth in the PC-3 tumor xenograft model as compared to control tumors. In other xenograft models, such as anaplastic thyroid cancer, ovarian cancer, and oral squamous cell cancer, inhibitory effects of CXCR4 antagonism on tumor growth and metastasis have been demonstrated [33]. Recently, several CXCR4 antagonists have been developed to block the SDF-1/CXCR4 axis and are at different stages of development [34]. The first-in-class CXCR4 antagonist, plerixafor (AMD3100), was approved by the United States Food and Drug Administration in 2008 for the mobilization of hematopoietic stem cells. Several other drugs are also currently in clinical trials. CXCR4 antagonists such as plerixafor, TG-0054, AMD070, MSX-122, CTCE-9908, and POL6326 are under investigation in phase I/II clinical trials for patients with cancer, human immunodeficiency virus, and myelokathexis [34].
The current meta-analysis provides further evidence of the relationship between CXCR4 expression and metastasis in prostate cancer. Increased CXCR4 expression in prostatectomy specimens could be a useful predictor of poor prognosis, with a relatively high probability of metastasis or the future development of metastatic disease. In addition, preclinical studies have suggested that blocking the SDF-1/CXCR4 interaction alone or in combination with other therapeutic modalities might be a potential strategy for metastatic prostate cancer. Taken together, results from phase I/II clinical trials evaluating efficacy and data regarding the safety of the available CXCR4 antagonists are promising for patients with advanced prostate cancer.
The present meta-analysis showed that increased CXCR4 protein expression in prostate cancer specimens is significantly associated with the presence of metastatic disease. However, CXCR4 expression was not associated with Gleason scores or T stage. Our meta-analysis results strongly support previous experimental data highlighting the role of the SDF-1/CXCR4 axis in prostate cancer metastasis.
Figures and Tables
Fig. 1
Study selection flow chart. The full texts of articles were reviewed, and 11 articles were selected as potential candidates for the meta-analysis. Subsequently, six articles that did not fit the eligibility criteria of this meta-analysis were removed. Finally, five articles were included in the analysis of the relationship between CXCR4 and the clinicopathological features of prostate cancer.

Fig. 2
Forest plot of high versus low expression of CXCR4. (A) There is no relationship between CXCR4 expression and Gleason scores (GS; <7 vs. ≥7) according to the meta-analysis. (B) CXCR4 expression is not associated with T stage (<T3 vs. ≥T3), and the relevant meta-analysis showed an odds ratio (OR) of 1.803 (95% confidence interval (CI): 0.756~4.297; p=0.183). (C) Higher CXCR4 expression was strongly associated with the presence of metastatic disease, with a fixed-effects pooled OR of 7.459 (95% CI: 2.665~20.878; p<0.001). W: weight.

Fig. 3
Radial plots indicated no heterogeneity after selection of effects models for all studies. CXCR4 expression and Gleason score (A), CXCR4 expression and T stage (B), and CXCR4 expression and metastasis (C).

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant from the Yonsei University College of Medicine for 2010 (6-2010-0070).
References
1. Lee JY, Lee DH, Cho NH, Rha KH, Choi YD, Hong SJ, et al. Charlson comorbidity index is an important prognostic factor for long-term survival outcomes in Korean men with prostate cancer after radical prostatectomy. Yonsei Med J. 2014; 55:316–323.

2. The Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med. 1984; 311:1281–1286.
4. Briganti A, Suardi N, Gallina A, Abdollah F, Novara G, Ficarra V, et al. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev. 2014; 40:3–11.

5. Wells TN, Power CA, Lusti-Narasimhan M, Hoogewerf AJ, Cooke RM, Chung CW, et al. Selectivity and antagonism of chemokine receptors. J Leukoc Biol. 1996; 59:53–60.

7. Wang J, Wang J, Sun Y, Song W, Nor JE, Wang CY, et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005; 17:1578–1592.
8. Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006; 66:32–48.

9. Singh S, Singh UP, Grizzle WE, Lillard JW Jr. CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab Invest. 2004; 84:1666–1676.

10. Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005; 65:9891–9898.
11. Wang Q, Diao X, Sun J, Chen Z. Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate cancer cell line. Cell Biol Int. 2011; 35:897–904.

12. Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004; 18:1240–1242.

13. Mochizuki H, Matsubara A, Teishima J, Mutaguchi K, Yasumoto H, Dahiya R, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004; 320:656–663.

14. Xing Y, Liu M, Du Y, Qu F, Li Y, Zhang Q, et al. Tumor cell-specific blockade of CXCR4/SDF-1 interactions in prostate cancer cells by hTERT promoter induced CXCR4 knockdown: A possible metastasis preventing and minimizing approach. Cancer Biol Ther. 2008; 7:1839–1848.

15. Jung SJ, Kim CI, Park CH, Chang HS, Kim BH, Choi MS, et al. Correlation between chemokine receptor CXCR4 expression and prognostic factors in patients with prostate cancer. Korean J Urol. 2011; 52:607–611.

16. Okera M, Bae K, Bernstein E, Cheng L, Lawton C, Wolkov H, et al. Evaluation of nuclear factor κB and chemokine receptor CXCR4 co-expression in patients with prostate cancer in the Radiation Therapy Oncology Group (RTOG) 8610. BJU Int. 2011; 108:E51–E58.

17. Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008; 99:539–542.

18. de Muga S, Hernández S, Salido M, Lorenzo M, Agell L, Juanpere N, et al. CXCR4 mRNA overexpression in high grade prostate tumors: lack of association with TMPRSS2-ERG rearrangement. Cancer Biomark. 2012-2013; 12:21–30.

19. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

20. Keaney M, Lorimer AR. Auditing the implementation of SIGN (Scottish Intercollegiate Guidelines Network) clinical guidelines. Int J Health Care Qual Assur Inc Leadersh Health Serv. 1999; 12:314–317.
21. Flay BR, Biglan A, Boruch RF, Castro FG, Gottfredson D, Kellam S, et al. Standards of evidence: criteria for efficacy, effectiveness and dissemination. Prev Sci. 2005; 6:151–175.

22. Kumpfer KL, Alvarado R. Family-strengthening approaches for the prevention of youth problem behaviors. Am Psychol. 2003; 58:457–465.

23. Bröning S, Kumpfer K, Kruse K, Sack PM, Schaunig-Busch I, Ruths S, et al. Selective prevention programs for children from substance-affected families: a comprehensive systematic review. Subst Abuse Treat Prev Policy. 2012; 7:23.

24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

26. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007; 28:105–114.

27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.

28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

29. Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005; 23:879–894.

30. Wirth MP. Hormone-refractory prostate cancer: what have we learned? BJU Int. 2007; 100:Suppl 2. 56–59.

31. Cereda V, Formica V, Massimiani G, Tosetto L, Roselli M. Targeting metastatic castration-resistant prostate cancer: mechanisms of progression and novel early therapeutic approaches. Expert Opin Investig Drugs. 2014; 23:469–487.

32. Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, et al. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012; 14:709–718.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


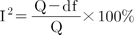


 XML Download
XML Download