INTRODUCTION
Heat Shock Proteins (HSPs), originally identified as stress-responsive proteins, are the most prominent group of proteins involved in folding and unfolding of other proteins. HSPs are found virtually in all living organisms from bacteria to humans [1]. Hsp60, Hsp70, and Hsp90 (the most widely studied HSPs) refer to families of HSPs in the order of 60, 70, and 90 kilodaltons in size, respectively [2]. HSPs represent a highly conserved class of cytoprotective proteins specifically induced at the cellular level in response to one of several environmental stressors (heat shock, cellular energy depletion, oxidative stress or inflammation amongst other).
Some of the important factors involved in HSP induction are hyperthermia, oxidative stress, inflammation, cellular damage (via calcium overload), hypoxia and ischemia and cellular energy depletion [3]. In contrast, others HSPs are constitutively expressed at normal temperature and are only slightly induced by heat shock. They play a crucial role in regulating apoptosis. Hsp27, Hsp70 and Hsp90 are considered to be anti-apoptotic, since they were able to bind to some pro-apoptotic molecules, including cytochrome c and apoptotic protease activating factor 1(Apaf 1) [4]. In contrast, Hsp10 and Hsp60 are considered to be pro-apoptotic [5], although Chandra et al [6] have described a dual role and underlying mechanism for Hsp60 (i.e. Pro-apoptotic and Pro-survival).
The functional diversity of the HSPs is indicative of their significance as biological molecules that play a significant role in the human aging and in male fertility. This review focuses on identifying the marker HSPs for aging and male fertility in humans.
Hsps 27, 60, 70 AND 90 ISOFORMS, AND SPERMATOGENESIS
Aging significantly alters male reproductive functioning and brings involutive changes in the testes and other organs. The effect of aging on the mammalian male reproductive organs has been principally analyzed in the testes. Spermatogenesis and steroidogenesis decrease with old age [7] and apoptosis increases with age, producing an accelerated germ cell loss [8]. HSPs are involved in the different developmental stages of spermatogenesis all of which involve dramatic transformations and cellular differentiation [9]. In testes showing maturational arrest, Hsp27 expression was strong Sertoli cells, weak in the spermatogonia and spermatocytes and absent in spermatids and Leydig cells. Adly et al [10] reported the differential expression patterns of Hsp27 in the human testes during normal spermatogenesis, indicating a possible role in this process. The seminiferous epithelium of human testes during normal spermatogenesis shows a cell type-specific expression of Hsp27, such that its expression is strong in the cytoplasm of the Sertoli cells, spermatogonia, and Leydig cells, moderate expression was observed in the spermatocytes, weak in the spermatids and absent in the spermatozoa. The altered expression of Hsp27 in testes showing abnormal spermatogenesis may be related to the pathogenesis of male infertility. Hsp60 has been detected in spermatogonia, primary spermatocytes, and Sertoli cells in the rat [11] and human testes [12]. HSPs have been detected on the surface of mouse, rat, bull, boar, and human sperm, and Hsp70 family members appear to be abundant components of the sperm surface [13,14]. Data from mouse, rat, bull, and indicate that Hsp70 protein is constitutively expressed in specific spermatogenic cell types during spermatogenesis [15,16]. Deficiency of Hsp70-2 in mice results in the arrest of spermatogenesis in meiotic prophase I and leads to infertility [17]. Two isoforms of Hsp90 have been identified in the mouse and human [18] and are highly expressed in pre-meiotic spermatogenic cells [19]. In a recent study [20], it was reported that the aberrant expression of Hsp70 could disturb male fertility. There were significantly different expression levels of Hsp70-2 among normal, maturationally arrested and Sertoli cells-only testis tissues. Low expression of Hsp70-2 was detected in maturationally arrested testes compared to normal testes and Hsp70-2 could not be detected in Sertoli cell-only testes [21]. The Hsp70 proteins are among the most conserved proteins known and are highly homologous from bacteria to man [22].
Rong et al [23], have demonstrated differential Hsp90 expression in mouse testis and epididymis. Hsp90 is expressed in the mouse [24], pig [15], and rat [25] testes. Lee [18] has demonstrated high levels of Hsp90 transcripts in mouse meiotic prophase spermatogenic cells. Hsp90 is expressed at them spermatozoal surface and becomes tyrosine-phosphorylated during sperm capacitation [26]. Wu [27] showed that the expression pattern of Hsp90 in the rabbit testes was similar to that of Hsp70. Liu et al [28] examined Hsp90 alpha in the testicular biopsy specimens of 57 infertile men with maturational arrest. Hsp90 alpha is expressed in spermatogonia, spermatocytes, Sertoli cells, Leydig cells, and myoid cells in the normal testicular tissues, but highly expressed in the testicular tissues with maturational arrest.
ROLE OF HEAT SHOCK PROTEIN IN AGING
Assorted theories state that the length of life is related to an organism's resistance to intrinsic and extrinsic stress, which indicates that the life span is stress-dependent [29]. HSPs are not only induced in response to intrinsic and extrinsic stressors but also are the significant mediators of an organism's resistance to stress. This resistance is associated with promotion of an HSP gene, which in turn activates the HSP expression during aging, enhancing stress resistance and extending the life span. During aging, HSPs show tissue and disease specific expression patterns in unstressed aging animals [30,31]. The expression of HSP chaperones has a significant role in aging and longetivity. An unbalanced chaperone system in an aged organism can lead to the accumulation of aggregated proteins resulting mostly in folding diseases in the nervous system. Therefore, over-expression of chaperones often delays the onset or diminishes the symptoms of the disease [32], and increased chaperone induction can lead to increased longevity [33]. HSPs are increasingly being implicated in aging phenotypes and the control of life dyad across species [34].
Jonak et al [35] indicated a link between Hsp27 expression and age-dependent epidermal alterations. High levels of HspB1 (Hsp27) expression usually induces a protective pro-reducing state characterized by a decrease in the levels of reactive oxygen species (ROS) resulting in an HspB1-mediated up-regulation of reduced glutathione and mitochondrial membrane potential [36,37]. The anti-oxidative potential of HspB1 is therefore crucial to counteract the oxidative damages associated with many neurodegenerative [38] and inflammatory diseases, such as asthma induced airway inflammation [39]. During a stressed cell state, Hsp27 functions as a chaperone, while in an unstressed state; it is thought to provide cytoskeletal structural stability [40].
Hsp27 has been implicated in various neurodegenerative diseases. A highly induced expression of Hsp27 has been reported in the brains of aged persons and those with Alzheimer's disease (AD) patients. Hsp27 is present in proliferating astrocytes, neurofibrillary tangles, and Hirano bodies, some hippocampal neurons are also Hsp27 positive. However, in control brains Hsp27 immunoreactivity is restricted to blood vessels and to occasional astrocytes in the white matter. Similarly, patients suffering from other types of dementia (Parkinson/dementia complex, multiinfarct dementia, normal pressure hydrocephalus) showed a certain degree of expression Hsp27 in reactive astrocytes, but that was less than that in AD but more than that in controls. Occasional Hsp27 immunoreactive astrocytes were present in cases without dementia (parkinson's disease, lacunar state, or focal ischemic necrosis) [41].
Dysfunction of chaperones indicates deterioration in the quantity and quality and in addition to physical degeneration. Indentifying the role of Hsp during aging is not simple as aging is accompanied by a number of diseases [42]. Proteins of this family are also called chaperonins. Hsp70 gene polymorphism is observed individuals with shorter lifespan and induction of the Hsp70 gene has been observed in heat- treated isolated blood cells [43]. These results supports the hypothesis that increased longetivity is correlated with lower Hsp gene expression.
The HSP90 Protein family Hsp90 is important in the formation of steroid receptor complexes. To examine the role of chaperones in aging a stochastic model of the chaperone Hsp90 was developed [44]. Hsp90 constitutes upto 2% of the total number of cellular proteins and is highly conserved and abundant molecule [45]. Hsp90 is involved in aging. During the aging process, the normal increase in the concentration of Hsp90 in response to thermal stress is attenuated in lymphocytes [46], liver cells [47], and mesenchymal stem cells [48]. Diminished Hsp90 messenger RNA and protein synthesis following mitogen stimulation of lymphocytes with interleukin-2 has been observed in T lymphocytes from aged donors [49], and tissue from aged animals and blood from elderly humans show reduced production of stress proteins, including Hsp90, following thermal stress [50]. Hsp90 is further implicated in altered homeostasis of aging, which may be particularly relevant in cartilage due to the requirement of this tissue for accurate telomerase assembly and function [51] and given the loss of telomere length with age in chondrocytes [52].
There is increasing evidence for the involvement of HSPs and related heat shock factors in male-derived infertility. HSPs have been reported to be up-regulated in oligospermia and varicocele cases [53]. Several studies have strongly indicated that Ctehlamydia trachomatis may impair fertility by damaging the function of HSPs on the surface of spermatozoa [54].
HEAT SHOCK PROTEINS IN APOPTOSIS
Earlier reports have suggested that mitochondria can be affected by heat tension [55], and data from yeast [56], Antarctic bivalves [57], and rat cardiomyocytes [58] suggest that severe heat stress can structurally and functionally alterations in mitochondria. Left unchecked, dysfunctional mitochondria can cause cell death and eventually lead to deficits in organ function [59]. Haak et al [60] have stated that aging-related stultification of the mitochondrial stress response might have a broad negative influence on the power of aged organisms to tolerate physiological stress.
Aging-related impairments of the mitochondrial stress response may have a broad negative influence on the ability of aged organisms to tolerate physiological stress. Drosophila aging is characterized by a small but widespread downregulation of mitochondrial metabolism and electron transport chain genes [61,62], and this pattern is also observed in aging mammalian tissues [63]. Sustained oxidative damage to nucleic acids, proteins and lipids caused by ROS, is considered to be a major factor in the general functional decline of tissue associated with aging and age-associated degenerative diseases [64,65]. With age, the fluidity of cell membranes, those of mitochondria, decreases and this is associated with enhanced lipid peroxidation [66].
The main mitochondrial stress proteins are Hsp60 and mtHsp70 (mortalin) [67], all of which perform the vital functions of importing, transporting, refolding, and preventing aggregation of mitochondrial proteins [67,68,69]. Hsp60 is the main heat-inducible protein, although the expression of all three proteins can be upregulated during mitochondrial and cellular perturbation. It has been shown previously that mitochondrial protein degradation and import, two key functions of mitochondrial stress proteins, are impaired with aging, implying that the mitochondrial stress response may be diminished in older organisms [69,70].
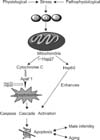
While hyperthermic challenge has been shown to induce apoptosis in young mice and rats [71,72], the high levels of cytochrome c release observed in a study [60] suggest that there is a strong activation of the apoptotic caspase cascade in older organisms. Additionally, the blunted Hsp60 levels in older mitochondria may contribute to an apoptotic response after a challenge, as this mitochondrial stress protein has been reported to play a role in suppressing apoptosis [69]. The release of cytochrome c, along with the decreased protein levels of Hsp60, may combine to promote apoptosis in aged animals after a stress induced disruption of normal function.
Mitochondrion have a key role in apoptosis since many of the endogenous cellular proteins that function as crucial determinants of cell death bring about their anti-apoptotic abilities by acting on mitochondria, thereby helping to prevent release of crucial pro-apoptotic proteins [73]. Experiments have demonstrated that Hsp72 and Hsp27 increase cell survival in response to apoptotic stimuli [74,75].
High temperatures can increase the rates of biochemical response which in turn can increase cell metabolism and might lead to increased oxidative processes. Levels of ROS have been shown to increase after exposure to both lethal (≥42℃) [76] and non-lethal (40℃) temperatures [77]. This might arise as a result of the mitochondrial respiratory chain dysfunction probably due to increased generation of ROS such as superoxide and hydrogen peroxide.
Cell death is an conserved evolutionary process characterized by a specific set of biochemical and morphological events, resulting in the ordered disassembly of the cell [78,79]. Caspase dependent apoptosis (Fig. 1) [80], occurs as molecular signaling cascade leading to the phenomenon of on blebbing. The resultant apoptotic cells are rapidly identified by phagocytic cells without induction of inflammation or tissue scarring [81].
Caspase-mediated cell death depends on activation of caspases that will then cleave a number of substrates [82] resulting in the biochemical and morphological changes typical of this kind of death. From a functional point of view we can distinguish two classes of caspases can be identified: upstream and downstream caspases. Activation of the up-stream caspases takes place when a sufficient number of enzyme molecules appear in finish adjacency and undergo conformational changes upon binding to the activation coordination compound, resulting in their cleavage and full activation [83]. Downstream caspases are activated by cleavage of the prodomain by upstream caspases. Two major molecular pathways lead to caspase activation and therefore to apoptosis the so-called extrinsic and intrinsic pathways. Multicellular organisms have evolved a series of molecular signaling events for responding to cellular damage and environmental strain. Included among these is the induction of a subfamily of HSPs designated as chaperonins (Hsp60, Hsp70, and Hsp90). Known functions of the chaperonins include folding, assembly and translocation of other proteins ,Since apoptosis is considered a normal and necessary a part of many physiological processes (e.g. development, cell turnover, cell injury), it follows that one important facet of the heat shock stress response would be to modulate the balance between cell expiry and survival [84]. HSPs are robustly induced in response to various stressors (both intrinsic and extrinsic) and are key mediators of an organism's resistance to stress [85]. HSPs have been shown to block apoptosis by interfering with caspase activation (Fig. 2). Over expression of Hsp27, Hsp70, Hsp60, or Hsp90, inhibits apoptosis and prevents caspase activation in many different cellular models following a variety of cellular stressors, including accumulation of misfolded proteins, or of ROS or DNA damage [86,87,88]. On the contrary, experimental depletion of Hsp27, Hsp60, Hsp70, or Hsp90, either by anti-sense constructions or siRNA strategies, increases the cells' sensitivity to apoptotic stimuli [89,90,91]. In some cellular contexts, Hsp70 exhaustion is sufficient to trigger apoptosis by activating caspase-3, in the absence of any additional stressful input [92].Therefore, HSPs are either directly or indirectly involved in the regulation of caspase activity.
Hsps can block both the intrinsic and the extrinsic apoptotic pathways through the interaction with keystone protein s at three points: (i) upstream of mitochondria, thereby modulating signaling nerve pathways; (ii) at the mitochondrial level, controlling the release of apoptogenic particles; and (iii) and at the post-mitochondrial level, by blocking apoptosis at a later stage than any known survival enhancing drug or protein. HSPs therefore playing an important role in the maintenance and survival potential of cells by acting as anti-apoptotic proteins, a function that appears to be independent of their chaperoning activity. The death-inducing signaling complex is formed as a result of ligand binding to specific receptors in the intrinsic pathway, and caspases-8 is subsequently activated. In the intrinsic pathway, the release of cytochrome c from the mitochondria results in the formation of the apoptosome and activation of caspase-9. Downstream caspases such as caspases-3 are activated by caspase- 8 and caspases-9, resulting in cell death. The two pathways are connected through the cleavage of the BH3 only protein; BH3 interacting domain (BID) [81].
Recent evidence has demonstrated that a significant pool of Hsp27 is located in the mitochondrial fraction of thermotolerant Jurkat cells [75]. Hsp27 is devoid of any endogenous ROS detoxifying activity, and can increase intracellular levels of glutathione [74]. It is a tripeptide with numerous functions within cells, including ROS detoxification and regulation of cell death.
Hsp70 also sequesters released apoptosis-inducing factor (AIF) from the mitochondria, thereby preventing caspase independent cell death [93]. Hsp27 and Hsp70 can modulate the death receptor pathway of apoptosis by hindering tBid translocation to mitochondria, which in turn inhibits cytochrome c release [94]. Hsp27, Hsp70, and Hsp90 can inhibit apoptosis upstream of mitochondria [95] and interfere with apoptosome formation, post-mitochondrial events, and caspase activation [96]. Furthermore, Hsp70 and phosphorylated Hsp27 can protect cells against oxidative stress. Hsp90 has been shown to be a negative regulator of caspase-2 activation [97]. Furthermore, Hsp70 and phosphorylated Hsp27 can protect cells against oxidative stress. Hsp90 has been shown to be a negative regulator of caspase-2 activation [97].
Hsp27 and Hsp70 can act at multiple control points of the apoptotic pathways to ensure that stress-induced damage does not inappropriately trigger cell death. Hsp70 and Hsp27 acts as endogenous inhibitors of apoptosis. Hsp70 is a decisive negative regulator of the mitochondrial pathway of caspase-mediated cell death that can block apoptosis at different stages, at a pre-mitochondrial stage by inhibiting stress-inducing signaling; at the mitochondrial stage by preventing mitochondrial membrane permeabilization through the occlusion of Bax translocation; and, at the post-mitochondrial level by interacting with AIF and Apaf-1 or by protecting essential nuclear protein from caspase-3 cleavage. Hsp27 can block cytochrome c induced caspase activation at different stages, namely at the pre-mitochondrial level by inhibiting cytochrome c waiver indirectly through its actions on F-actin, Bid or ROS and at the post-mitochondrial level through the sequestration of cytosolic cytochrome c. Hsp27 may also influence apoptosis by supporting the ubiquitination or degradation of proteins like IkBa or p27kip1 under stressful conditions. Hsp70 can also block caspase-independent pathways, because Hsp70 prevents cell death in conditions in which caspase activation does not occur [98]. In stressed cells, Hsp90 can bind to Apaf-1 thereby inhibiting the activation downstream [99]. Molecular chaperones of the Hsp90 gene family are considered indispensable regulators of protein folding.
Of the molecular chaperones regulated by heat shock factor 1, Hsp70 is the best understood regarding its regulation of signaling and cell death pathways. In addition to its role in promoting the refolding or clearance of misfolded or aggregated proteins, Hsp70 prevents apoptosis through its direct binding with Apaf1 [100].
CONCLUSION
The available literature indicates a clear expression and functional role of the HSPs during spermatogenesis or and process of aging without which the related mechanisms may alter. HSPs play an important role in apoptosis in mitochondrially-mediated aging and male infertility. Hsp27 and Hsp70 are found to be anti-apoptotic while Hsp60 and Hsp90 are pro-apoptotic. The differential expression of HSPs during the various stages of spermatogenesis in species from Drosophilla to human indicates significant role for Hsp27, 60, 70, and 90 in male infertility and also their prompt role in the apoptosis. Mitochondria are the main target site for apoptosis, which in turn leads to aging of the somatic cells and decreased fertility in a germ cells. Consequently, the differential expression of HSPs can be studied with the progression of age. Therefore, Hsp27, Hsp70, Hsp60, and Hsp90 can be used as the molecular markers of mitochondrially- mediated aging and male infertility.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download