Abstract
Purpose
To investigate the relationships among the Wnt/β-catenin pathway, androgen receptor (AR), and clinicopathological factors in hormone-naïve prostate cancer.
Materials and Methods
This study was conducted with132 cases of hormone-naïve prostate cancer treated by prostatectomy and prostate needle biopsy. An immunohistochemical study using antibodies against β-catenin, matrix metalloproteinase-7 (MMP-7), and the AR was performed. For the in vitro study, PC-3, LNCaP, 22Rv1, and DU145 cell lines were used.
Results
The clinical or pathological stage ware a localized cancer in 36 patients (27.3%), locally advanced cancer in 31 (23.5%), and metastatic cancer in 65 (49.2%). We detected increased β-catenin, AR, and MMP-7 expression with a high Gleason grade, disease progression, and increasing serum prostate-specific antigen (PSA) levels (p<0.01). In Spearman's rank correlations, the expression of cytoplasmic β-catenin, MMP-7, and the AR were found to be significantly positively correlated. In addition, the expression of β-catenin, MMP-7, and the AR were significantly correlated with clinicopathological variables indicative of a poor prognosis. Forty-nine patients with primary androgen deprivation had short response durations from hormone therapy to PSA progression with elevated MMP-7 expression on the Kaplan-Meier curve (p=0.0036).
Conclusions
These data show that an activated Wnt/β-catenin pathway and AR expression in prostate cancer are correlated with metastasis and aggressiveness. In addition, the expression of MMP-7 protein, a target of the Wnt/β-catenin pathway, is associated with PSA progression in prostate cancer patients undergoing primary hormone therapy.
Wnt/β-catenin signalling plays a fundamental role in controlling a variety of cellular processes, such as the determination of cell fate, cell proliferation, migration, and polarity, and the maintenance of stem cells. Many studies have reported that changes in Wnt signalling can lead to carcinogenesis and the progression of malignancies, including prostate cancer.1-3 In addition, some investigators have suggested that Wnt/β-catenin signalling and the androgen receptor (AR) play critical roles in prostate cancer progression.1,4,5
In the absence of Wnt signals, β-catenin is ubiquitinated and degraded by the proteasome pathway, resulting in low levels of cytoplasmic β-catenin. The activation of Wnt signalling prevents the ubiquitination of β-catenin and elevated cytoplasmic and nuclear β-catenin levels up-regulate target genes like cyclic D1 and c-myc. Recent studies have shown that β-catenin plays an important role in the expression of matrix metalloproteinases (MMP)-7 and the AR and alters cancer cell characteristics.1,4,6
Degradation of the extracellular matrix mediated by MMPs is a crucial step in tumour invasion and metastasis of various cancers. MMP-7 has been identified as a target of the Wnt/β-catenin pathway, and MMP-7 overexpression is strongly associated with the nuclear/cytoplasmic accumulation of β-catenin in cancer.2,3,7
Androgens play an important role in the growth, differentiation, and changes in prostate cancer. Their effects are mediated via the AR. Recently, investigators have found evidence of a relationship between Wnt/β-catenin and androgen signalling.1,5 The authors explained that Wnt/β-catenin and androgen signalling work separately in different cells under normal conditions. However, Wnt/β-catenin signalling affects AR signalling in cancer, especially androgen-independent prostate cancer. β-catenin interacts with AR and cytoplasmic β-catenin translocates into the nucleus with AR in the presence of androgens.5
To investigate the role of the Wnt/β-catenin pathway and AR in prostate cancer progression, we evaluated the expression of β-catenin, MMP-7, and AR by immunohistochemistry and in vitro cell culture.
After obtaining Institutional Review Board approval (10-130), we retrospectively reviewed 132 prostate cancer patients who underwent radical prostatectomy (45 [34.1%]) or hormone therapy (87 [65.9%]) in the Department of Urology from October, 2003 to September, 2007 (Table 1). In our centre, prostate cancer is diagnosed from a transrectal ultrasound-guided biopsy in all patients following the same protocol. All clinical and clinicopathological data, such as age, preoperative prostate-specific antigen (PSA) level, Gleason grade (GG; the primary grade was adopted), and TNM stage, were obtained from medical records. Staging was based on the 2002 TNM classification. All specimens were hormone-naïve prostate cancer tissues (radical prostatectomy and prostate biopsy specimens). The PSA nadir was defined as the lowest PSA value during androgen-deprivation therapy.8,9 PSA progression was defined as an increase ≥25% of the nadir and ≥2 ng/ml above the nadir.10
Immunohistochemistry for β-catenin (1:200, 610153, BD Biosciences, San Jose, CA, USA), MMP-7 (1:3000, M8683, Sigma, Saint Louis, MO, USA), and AR (1:10, sc-7305, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was performed on 4 µm thick paraffin-embedded prostate tissue sections. Briefly, all tissue sections underwent microwave antigen retrieval pre-treatment. Detection was performed using the Dako REAL™ EnVision Kit (Dako, Carpinteria, CA, USA), according to the manufacturer's instructions. 3-amino-9-ethyl carbazole (Immunotech, Foster City, CA, USA) was used as a chromogen and counterstaining was done with Mayer's haematoxylin (Dako). Negative control staining was done without application of the primary antibody.
The nuclear expression of AR and MMP-7 was scored by evaluating the staining intensity in cases with positivity in more than 10% of the cancer cells. Membranous and cytoplasmic β-catenin expression levels were determined separately. The staining intensity was evaluated semiquantitatively using the following criteria: 0, no staining or less than 10% positivity; 1, weak staining; 2, moderate staining; and 3, strong staining. An uropathologist (Pathologist: Soojin Jung) with no knowledge of the clinical data assessed the intensity of immunoreactivity in the cells.
The human prostate cell lines-22Rv1, LNCaP, PC-3, and DU145 and cell lines for conditioned medium (CM; L cells and L cells that secrete Wnt3a) were maintained routinely in RPMI-1640 or Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (FBS), 120 µg penicillin/ml, and 200 µg streptomycin/ml. L-cell (CM) and Wnt3a-conditioned medium (Wnt3a-CM) was prepared by culturing Wnt3a-secreting L cells in DMEM with 10% FBS for 4 days. The medium was harvested and sterilised using a 0.22 µm filter. Fresh medium was added and the cells were cultured for a further 3 days. The medium was again collected and combined with the previous medium.
First, 22Rv1, PC-3, and DU145 cells were cultured overnight in six-well plates with CM and Wnt3a-CM. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Isolated RNA was reverse transcribed (c reverse transcription System; Promega, Madison, WI, USA), and 1 µl of cDNA was subjected to 35 cycles of the PCR. β-actin mRNA was used as a control (30 cycles). PCR primers were as follows: hMMP1 forward, CGA CTC TAG AAA CAC AAG AGC A; hMMP1 reverse, AAG GTT AGC TTA CTG TCA CAC GCT T; hMMP2 forward, CTG ACA TTG ACC TTG GCA CC; hMMP2 reverse, TAG CCA GTC GGA TTT GAT GC; hMMP3 forward, CAG GTG TGG AGT TCC TGA T; hMMP3 reverse, AGG TTC TGG AGG GAC AGG TT; hMMP7 forward, GGT CAC CTA CAG GAT CGT ATC ATA T; hMMP7 reverse, CAT CAC TGC ATT AGG ATC AGA GGA A; hMMP9 forward, TCT TCC CTG GAG ACC TGA GA; hMMP9 reverse, CAC CAA ACT GGA TGA CGA TG; hMMP14 forward, CGC TAC GCC ATC CAG GGT CTC AAA; and hMMP14 reverse, CGG TCA TCA TCG GGC AGC ACA AAA.
LNCaP and 22Rv1 cells were cultured in glass chamber slides with CM and Wnt3a-CM for 1 h. The cells were washed with PBS, fixed with 4% formaldehyde, permeabilised in 0.3% Triton X-100, and blocked in 4% bovine serum albumin for 1 h. The cells were stained with anti-β-catenin (610153, BD Biosciences), anti-AR (sc-7305) antibodies, and DAPI (4',6-diamidino-2-phenylindole), and then analysed using a Zeiss Axioplan 2 fluorescence microscope equipped with a Zeiss Axiocam utilising Openlab software (Improvision, PerkinElmer, Waltham, MA, USA).
Statistical calculations (SPSS ver. 11.5, SPSS, Chicago, IL, USA) consisted of the chi-squared test, Fisher's exact test, linear-by-linear association, analysis of variance (ANOVA) with the least significant difference (LSD), and Spearman rank correlation coefficient analysis. The Kaplan-Meier curve was used to show the relationship between PSA progression and the expression level of each protein. A value of p<0.05 was considered to indicate statistical significance.
The mean patient age at diagnosis was 70.5±7.3 (49~84) years. The clinical or pathological stage was localised cancer in 36 patients (27.3%), locally advanced cancer in 31 (23.5%), and metastatic cancer in 65 (49.2%). The mean initial PSA was 291.2±932.3 ng/ml (2.63~7337) (Table 1).
To evaluate the relationship between the Wnt pathway and AR, we determined the locations of β-catenin and AR in Wnt3a-CM. Immunofluorescence analysis using anti-β-catenin and anti-AR antibodies showed that β-catenin and AR were detected primarily in the cytoplasm with an inactivated Wnt pathway. By contrast, with Wnt3a-CM, the nuclear accumulation of β-catenin and AR was observed in LNCaP and 22Rv1 cells (Fig. 1A). To determine the β-catenin target genes from among several MMPs, we screened MMP-1, -2, -3, -7, -9, and -14. After 35 cycles of RT-PCR, activation of the canonical Wnt pathway (Wnt3a-CM) led to an increase in MMP-7 mRNA in prostate cancer cells (Fig. 1B).
β-catenin was expressed in the cytoplasm and membranes of cancer cells. Of the cancer cells, 93.9% (124/132) showed membranous β-catenin staining and 72.0% (95/132) were cytoplasm-positive (Fig. 2A). The expression of cytoplasmic β-catenin was significantly correlated with a high GG, high preoperative PSA, disease progression, and bone metastasis (p<0.01) (Fig. 2B~E). Of the 132 patients, nucleus-positive cells were identified in four (data not shown).
Spearman's rank correlations were used to explore potential associations among β-catenin, MMP-7, and AR expression levels, and other clinicopathological variables (Table 2). In this analysis, the expression of cytoplasmic β-catenin, MMP-7, and AR were found to be significantly positively correlated. In addition, the expression of β-catenin, MMP-7, and AR were significantly correlated with clinicopathological variables indicative of a poor prognosis (high preoperative PSA, high primary Gleason score, and bone metastasis).
Of 87 patients on primary hormone therapy, 49 who were followed for at least 1 year were enrolled. Prostate cancer patients with moderate-to-strong MMP-7 expression had a short duration to PSA progression compared to those with negative-to-weak expression (log-rank test, p=0.036). The expression of β-catenin and AR did not affect PSA progression (Fig. 5).
We investigated the expression of Wnt pathway proteins and the AR in prostate cancer. There were significant relationships among the expression of β-catenin, MMP-7, and AR and the clinicopathological variables in prostate cancer.
A hallmark of the activation of Wnt signalling is stabilisation of β-catenin. Traditionally, the role of β-catenin is to regulate cadherin-mediated intercellular adhesion. Since many studies have reported that β-catenin can function as an oncogene and is important in Wnt signalling, β-catenin is drawing attention in cancer research.1,11 Intracellular signalling including Wnt/β-catenin may be interactive or interconnected. Under normal conditions, cytoplasmic β-catenin is phosphorylated, binds to the APC/GSK3β/axin complex, and is degraded by proteasomes. Upon activation of Wnt signalling, β-catenin is not phosphorylated and accumulates in the cytoplasm. This results in the translocation of β-catenin into the nucleus and regulates the target gene. The activation core of the Wnt pathway in tumorigenesis is cytoplasmic or nuclear β-catenin. In our study, β-catenin was localised to the membrane and cytoplasm, although a few samples showed nuclear expression of β-catenin. We investigated primarily cytoplasmic β-catenin in human prostate cancer tissue of various stages. In this study, the cytoplasmic expression of β-catenin was correlated with tumour grade, cancer progression (stage), preoperative PSA level, and bone metastasis. Cytoplasmic β-catenin expression was moderate-to-strong in 19.4% of 36 cases of localised prostate cancer versus 35.5% of 31 cases of locally advanced prostate cancer and 60.0% of 65 cases of metastatic prostate cancer. This supports the hypothesis that changes in the Wnt pathway affect the progression of prostate cancer.
In general, we failed to identify nuclear expression of β-catenin in prostate cancer. Of 132 patients, positive nuclei were identified in only four (data not shown). Although we did not observe nuclear expression in most cases, because increasing cytoplasmic/nuclear β-catenin results in activation of the Wnt pathway, we postulated that cytoplasmic β-catenin might result in activation of the Wnt pathway.11-13 In colorectal and hepatocellular carcinoma, nuclear β-catenin is dominant, while studies have reported various expression patterns of β-catenin in prostate carcinoma.11,12
Consequently, we assessed MMP-7 as a target of β-catenin to identify activation of the Wnt/β-catenin pathway. MMPs are capable of degrading the extracellular matrix. Based on their architectural features, there are 23 MMPs in humans. For invasion or metastasis, cancer cells must cross extracellular barriers. In vivo and in vitro evidence indicates that MMPs play a role in metastasis.14,15 Among them, MMP-7 or matrilysin is a target gene of the Wnt/β-catenin pathway, which results in increased MMP-7 expression upon dephosphorylation of β-catenin or TCF/LEF activation.16 In our in vitro study, activation of Wnt/β-catenin signalling (with Wnt3a CM) led to increased MMP-7 mRNA expression in prostate cancer cell lines. In addition, the immunohistochemical expression of cytoplasmic β-catenin and MMP-7 were positively related according to the intensity of expression (linear-by-linear association p=0.043). Overexpression of MMP-7 is correlated with disease progression and metastasis in human cancer.6,17,18 In our hormone-naïve prostate cancer, MMP-7 was overexpressed in metastatic cancer compared to localised or locally advanced cancer. In addition, MMP-7 expression was significantly higher in patients with a high GG (GG=4~5) and high serum PSA (>20 ng/ml). Some previous reports support our findings. Hashimoto et al19 reported that the MMP-7 mRNA level increased with high pathological stages, histological differentiation, and serum PSA.
Intracellular signalling pathways are frequently interconnected, and Wnt/β-catenin and AR signalling are no exception. Some studies have suggested that β-catenin in prostate cancer is not restricted to the transcriptional activation of TCF/LEF, being also associated with the development of androgen sensitivity.1,5 β-catenin binds to the AR and acts as a co-activator thereof.5 Indeed, β-catenin preferentially binds to the AR, and the β-catenin/AR complex translocates into the nucleus in both a yeast two-hybrid system and the LNCaP cell line in the presence of androgens.20 Thus β-catenin interacts with the AR and enhances its transcription.21
The importance of the interaction of Wnt/β-catenin-AR signalling is its biological significance in the progression of prostate cancer. β-catenin is known to affect androgen sensitivity and the progression of prostate cancer.1,5,21 There was a significant positive relationship between cytoplasmic β-catenin staining and AR staining (Spearman's rank correlation coefficient 0.335, p<0.01). In addition, there was a significant increase in AR expression with high risk prostate cancer and progression (high GG (GG=4~5), metastasis, and preoperative serum PSA>20 ng/ml).
We evaluated the expression of cytoplasmic β-catenin/MMP-7/AR and the PSA progression in patients undergoing primary hormone therapy. Of 87 prostate cancers, we enrolled 49 patients that were followed-up for at least 1 year.
The PSA progression of prostate cancer patients with moderate-to-strong MMP-7 expression is more rapid than in those that exhibit negative-to-weak expression. There was no significant expression of cytoplasmic β-catenin and AR. High MMP-7 expression was related to the pathological stage and incidence of metastasis.19 However, there was no significant correlation with tumour progression and survival in localised prostate cancer.22 In our series, the pathological stage and expression of MMP-7 in localised prostate cancer were not correlated. A larger study should evaluate whether MMP-7 expression is a prognostic factor for androgen-deprivation therapy of prostate cancer. Our data are insufficient to draw conclusions on the functional association between Wnt/β-catenin and androgen signalling. However, our study of the Wnt/β-catenin-androgen pathway is helpful, since our findings provide important information regarding the interactions between the Wnt signalling pathway and AR during the progression of prostate cancer.
Figures and Tables
Fig. 1
In vitro, activation of the canonical Wnt pathway (Wnt3a-CM) led to translocation of β-catenin and the androgen receptor to the nucleus (A), and increased MMP-7 mRNA expression in prostate cancer cells (B). Wnt3a-CM: Wnt3a-conditioned medium, MMP: matrix metalloproteinase.
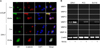
Fig. 2
Expression of β-catenin. β-catenin was expressed in both the membrane and cytoplasm of cancer cells (A: ×200). Cytoplasmic β-catenin expression was significantly correlated with a high Gleason grade (GG), high preoperative prostate-specific antigen (PSA), and disease progression (p<0.01) (B~D).
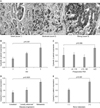
Fig. 3
Expression of matrix metalloproteinase-7 (MMP-7). MMP-7 was expressed in the nuclei of cancer cells (A: ×200). MMP-7 expression was significantly correlated with a high Gleason grade (GG), high preoperative prostate-specific antigen (PSA), and disease progression (p<0.01) (B~D).
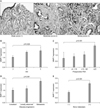
Fig. 4
Expression of the androgen receptor (AR). The AR was expressed in the nuclei of cancer cells and cytoplasm (A: ×200). AR expression was significantly correlated with a high Gleason grade (GG), high preoperative prostate-specific antigen (PSA), and disease progression (p<0.01) (B~D).
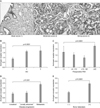
Fig. 5
Relationships among the expression of β-catenin, matrix metalloproteinase-7 (MMP-7), and androgen receptor (AR) and prostate-specific antigen (PSA) progression after primary hormone therapy. Cases with moderate-to-strong MMP-7 expression exhibited rapid PSA progression. (A) β-catenin, (B) MMP-7, (C) AR.

References
1. Wang G, Wang J, Sadar MD. Crosstalk between the androgen receptor and beta-catenin in castrate-resistant prostate cancer. Cancer Res. 2008. 68:9918–9927.
2. Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999. 18:2883–2891.
3. Saeki H, Tanaka S, Sugimachi K, Kimura Y, Miyazaki M, Ohga T, et al. Interrelation between expression of matrix metalloproteinase 7 and beta-catenin in esophageal cancer. Dig Dis Sci. 2002. 47:2738–2742.
4. Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009. 19:683–697.

5. Schweizer L, Rizzo CA, Spires TE, Platero JS, Wu Q, Lin TA, et al. The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC Cell Biol. 2008. 9:4.

6. Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005. 11:2117–2123.
7. Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999. 155:1033–1038.
8. Stewart AJ, Scher HI, Chen MH, McLeod DG, Carroll PR, Moul JW, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol. 2005. 23:6556–6560.

9. Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002. 168:995–1000.

10. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008. 26:1148–1159.

11. de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003. 9:1801–1807.
12. Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004. 101:1345–1356.
13. Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest. 2008. 88:1340–1348.
14. Szarvas T, Becker M, vom Dorp F, Gethmann C, Tötsch M, Bánkfalvi A, et al. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010. 101:1300–1308.

15. Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003. 36:128–137.

16. Robertson BW, Chellaiah MA. Osteopontin induces beta-catenin signaling through activation of Akt in prostate cancer cells. Exp Cell Res. 2010. 316:1–11.
17. Maruta S, Sakai H, Kanda S, Hayashi T, Kanetake H, Miyata Y. E1AF expression is associated with extra-prostatic growth and matrix metalloproteinase-7 expression in prostate cancer. APMIS. 2009. 117:791–796.

18. McDonnell S, Navre M, Coffey RJ Jr, Matrisian LM. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991. 4:527–533.

19. Hashimoto K, Kihira Y, Matuo Y, Usui T. Expression of matrix metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. J Urol. 1998. 160:1872–1876.

20. Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002. 277:11336–11344.
21. Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000. 60:4709–4713.
22. Boxler S, Djonov V, Kessler TM, Hlushchuk R, Bachmann LM, Held U, et al. Matrix metalloproteinases and angiogenic factors: predictors of survival after radical prostatectomy for clinically organ-confined prostate cancer? Am J Pathol. 2010. 177:2216–2224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download