Abstract
Purpose
Heat shock protein 27 (HSP27) is known as the material that plays a role in apoptosis control in tumor and cell protection including the immune response, drug tolerance, and so on. In this study, HSP27 expression according to the prostate cancer malignancy level was evaluated, and HSP27 expression was also analyzed after inducing apoptosis by doxazosin treatment of the prostate cancer cell lines.
Materials and Methods
Reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence staining of the HSP27 was implemented by the culture of RWPE-1, LNCaP, androgen-independent human prostate cancer cells (PC-3), and TSU-Pr1 cell lines. Analysis was separately conducted in the control group, control vector group treated by dimethyl sulfoxide, and groups treated with 10 µM or 25 µM doxazosin. The expression of HSP27 in RT-PCR and immunofluorescence staining was observed and evaluated after conversion to numerical values.
Results
In the RT-PCR results, depending on the cell type, LNCaP, TSU-Pr1 showed the highest HSP27 expression followed by PC-3, LNCaP and RWPE-1 in sequence. After doxazosin treatment, the expression detected by RT-PCR was stronger at a 25-µM doxazosin concentration compared to that at a 10-µM concentration, and the result was similar by immunofluorescence staining.
Conclusions
HSP27 expression increased depending on the prostate cancer cell line. This meant that HSP27 expression was related to the prostate cancer malignancy level. Additionally, the higher the treatment concentration in PC-3 was, the higher the HSP27 expression was. This result showed that doxazosin induced apoptosis of prostate cancer.
The proportion of prostate cancer among cases of all types of cancer in males in Korea is gradually increasing [1]. Therefore, it is important to characterize the progression of prostate cancer to castration-resistant prostate cancer (CRPC) to improve prostate cancer discovery and treatment outcomes. Rocchi et al [2] reported that heat shock protein 27 (HSP27) plays important roles in the progress to CRPC. HSP is known to be related to folding, activation, trafficking, and transcriptional activity of most steroid receptors including androgen receptor [3]. It is also known that HSP27 plays important roles in the apoptosis signal transmission process related to caspase-3: HSP27 interrupts cytochrome c secretion of thread granules in the procaspase-9 pathway, and it inhibits apoptosis by interrupting caspase-3 activation and apoptosome formation by acting on cytochrome c or procaspase-3 [4]. One study showed that HSP expression inhibited apoptosis, and the other previous studies found that HSP27 was related to the hormone resistance acquisition of the LNCaP cell line, which is a kind of a prostate cancer [5,6].
Doxazosin exerts, via an apoptotic-induced mechanism, a potent antigrowth effect in vitro against androgen-independent human prostate cancer cells (PC-3), DU-145, and LNCaP human prostate cancer cells, independently of its R1-adrenoceptor antagonism or the hormone sensitivity status of cells. The antitumor activity of doxazosin was confirmed in mice bearing PC-3-induced prostate cancer, where it displayed a significant inhibition of tumor growth [7]. Doxazosin was a potent and moderately selective R1B-adrenoceptor antagonist, showing in vitro antiproliferative activity in PC-3, DU-145, and LNCaP human prostate cancer cells at submicromolar concentrations, and also in in vivo antitumor activity in PC-3-induced subcutaneous tumors in mice [8].
In this study, the HSP27 expression was determined according to the extent of malignancy of prostate cancer. The HSP27 expression patterns were also analyzed after apoptosis was induced by treating prostate cancer cell lines with doxazosin.
We purchased RWPE-1, LNCaP, PC-3, and TSU-Pr1 cells from the American Type Culture Collection (Rockville, MD, USA). We used these cell line cultures for our experiments. All of the subjects were divided into three groups: a control group, control vector group treated by dimethyl sulfoxide (DMSO), and groups treated with 10 µM or 25µM of doxazosin.
RWPE-1, LNCaP, PC-3, and TSU-Pr1 were preserved in F12 nutrient medium containing 10% fetal bovine serum and penicillin (100 units/mL)/streptomycin (100 ng/mL) (Gibco BRL, Grand Island, NY, USA). The cells were placed in 6-well plates (Nalge Nunc International, Rochester, NY, USA) at a concentration of 1×106 cells per well and cultured at 37℃ in an atmosphere of 5% carbon dioxide for 24 hours before treatment. All of the experiments were repeated at least 3 times at every step. Immunohistochemical staining and reverse transcription polymerase chain reaction (RT-PCR) were performed.
DNA fragmentation analysis was performed to assess apoptosis in PC-3 treated with doxazosin. The cells were homogenized in lysis buffer (pH 8.0) consisting of 0.3 M Tris (hydroxymethyl) aminomethane (Tris-HCI), 0.1 M NaCl, 0.01 M ethylenediaminetetraacetic acid (EDTA), and 0.2 M sucrose. The homogenates were incubated on ice in 0.6% sodium dodecyl sulfate for 30 minutes and potassium acetate 0.035 M for 60 minutes at 65℃, and then centrifuged at 5,000×g for 10 minutes.
The supernatants were extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol), followed by chloroform-isoamyl alcohol (24:1, vol/vol). The nucleic acid in the aqueous phase was precipitated with doxazosin was a potent selective R1B-adrenoceptor at -70℃ overnight and collected by centrifugation at 14,000×g for 30 minutes. The pellets were resuspended in Tris-EDTA (TE) buffer (10 mM Tris-HCI, 1 mM EDTA; pH 8.0), incubated with DNase-free RNase (500 µg/mL) at 37℃ for 60 minutes, and re-extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol), followed by an equal volume of chloroform: isoamyl alcohol (24:1, vol/vol). DNA in the resulting supernatants was precipitated with 0.1 mL of 3 M sodium acetate and 2.5 mL of cold absolute ethanol at -70℃ for 60 minutes and collected by centrifugation at 14,000×g for 30 minutes. After removing the ethanol, the pellet was resuspended in 50 µL distilled water, and its DNA content was determined spectrophotometrically at 260 nm. Equal amounts of DNA samples (20 µg) were electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide staining.
RT-PCR was used to analyze the clusterin mRNA expression. TRIzol reagent (Gibco BRL) was used to isolate the total RNA according to the manufacturer's instructions. The final pellet was dried at room temperature and dissolved into a diethyl pyrocarbonate solution (Sigma, St. Louis, MO, USA). Spectrophotometry was used to determine the RNA concentration. One microgram of the total RNA with the cDNA Synthesis Kit (Roche, Mannheim, Germany) was used to produce cDNA. PCR was performed for clusterin in a total volume of 25 µL containing 0.25 mM deoxynucleotide triphosphates (dNTPs), 1.5 mmol/L MgCl2, 1U Taq DNA polymerase, 10 pmol of each sense and antisense primer, and distilled water. The following cycling patterns were used for 35 cycles in a GeneAmp 2400 thermocycler (Perkin-Elmer, Wellesley, MA, USA): initial denaturation, 5 minutes at 94℃; denaturation, 30 seconds at 94℃; annealing, 30 sesonds at 58℃; and elongation, 30 seconds at 72℃. The program was followed by a final elongation step for 5 minutes at 72℃. The primers used for clusterin PCR were 5'-AAGGAAATTCAAAATGCTGTCAA-3' (sense) and 5'-ACAGACAAGATCTCCCGGCACTT-3' (antisense).
The effect of doxazosin on apoptosis in PC-3 was analyzed using an in situ cell death detection kit (Roche Diagnostics, Penzberg, Germany) at the end of each culture period. The cells were washed with phosphate-buffered saline (PBS) and fixed with methanol at -20℃ for 15 minutes. After rinsing the cells with PBS three times, 50 µL triphosphate nick-end labeling (TUNEL) reaction solution was added, and the incubation continued at 37℃ for an hour in the dark. After rinsing with PBS, the cells were counterstained with Hoechst 33258 (Sigma) at room temperature for 10 minutes and mounted using mounting media for fluorescence (DakoCytomation, Glostrup, Denmark). The apoptotic index was calculated by counting the number of TUNEL-positive cells and dividing this by the total number of cells.
Doxazosin (Pfizer Inc., New York, NY, USA) was prepared at 25 M, the median lethal dose (LD50) [5] concentration, in 0.25% DMSO. Cultures at 80% confluence were changed to fresh media and treated with doxazosin or PBS containing 0.25% DMSO as a control. The cultures were collected for RT-PCR and DNA fragmentation analysis. Then these were fixed for TUNEL assay and immunocytochemistry after treatment.
The cells were harvested in the usual way. Trypsin was not used to detach adherent cells. The cells were rinsed with cation-free PBS, then EDTA was used in PBS. After rinsing, the cells were washed in TCM-N3 by centrifuging in 50 mL conical tubes for 10 minutes at 2,000 rpm. The supernatants were aspirated and resuspended in 1 mL (TCM-N3). The number of cells was counted using a hemocytometer (The ideal number of cells is about 5×105 per tube). One milliliter of the cell suspension was pipetted into each of the tubes, and centrifuged for 5 minutes at 1,500 rpm. Ten microliter of the supernatant was aspirated, and the cells were resuspended by vortexing the tube. Antibodies were dispensed into microcentrifuge tubes; with multicolor experiments, all the antibodies for one tube can be pre-mixed before addition to cells. After adding antibody to each tube, it was incubated for 15 minutes at room temperature. The cells were resuspended by vortexing the tube, and 1 mL of DPBS-N3 was added. Then the tubes were centrifuged at 1,500 rpm for 5 minutes. The supernatant was aspirated, the cells were resuspended by vortexing, and 0.5 mL of 1% formaldehyde was added to the PBS slowly. These were left for at least 1 hour in the formaldehyde solution before running them through the cytometer.
In mRNA expression densitograms for each cell type, the HSP27 expression level of RWPE-1, LNCaP, PC-3, and TSU-Pr1 were 100, 110, 125, and 158% of control, respectively (Fig. 1). In HSP27 immunofluorescence staining of each cell type, HSP27-positive cells showed an intensity of staining in RWPE-1, LNCaP, PC-3, and TSU-Pr1, increasing in that order (Fig. 2).
The HSP27 mRNA expression values in PC-3 measured by RT-PCR were 50, 60, 110, and 128% of control, respectively (Fig. 3).
The HSP27 mRNA expression was 2.2-fold higher at a 10-µM doxazosin concentration and 2.4-fold higher at 25 µM compared with the control.
In HSP27 immunofluorescence staining with each concentration of doxazosin, the HSP27-positive cells showed more intensity with 25 µM than with 10 µM doxazosin (Fig. 4). The TUNEL assay showed a similar pattern.
HSP27 protein was mostly expressed in the nuclei in cells that displayed the condensed nuclei and scant cytoplasm characteristic of apoptosis, and most of the cells expressing HSP27 in the nuclei were TUNEL-positive (Fig. 4).
HSP is an important protein in cell biology. It is well preserved during the evolution process; and it is created by various stressful stimuli including environmental stimuli such as heat, ultraviolet irradiation, and heavy metals and biological stimuli such as infection, inflammation, hypoxia, tissue damage, and tumors [3]. HSP expression not only increases inhibition of cell damage but also plays important roles in cell protection including apoptosis control and immune response control inside tumors [9]. The important role of HSP27 is to control the dynamic changes in actin and apoptosis. The apoptosis inhibition activity of HSP27 reacts to the cytochrome c/Apaf-1/dATP complex in the procaspase-9 pathway or to inhibit partially the connectivity among the Fas and Ask1 and Daxx proteins [10]. In addition, it is known that HSP27 inhibits cytochrome c secretion in thread granules of the procaspase-9 pathway; HSP27 also inhibits caspase-3 activation and apoptosome formation by reacting to cytochrome c or procaspase-3 [11]. HSP initiates danger signals by reacting to chaperone antigenic peptides in tumor cells or induces an immune reaction that protects from tumor cells. It can be identified that antigen-presenting cells from HSP-peptide complex were observed in dead tumor cells. Additionally, it is recognized that HSP helps cytotoxic T-lymphocytes improve the ability to treat and transmit antigens that are included in tumor cells [12].
Miyake et al [13] reported that while HSP27, HSP70, HSP90, etc. showed a reaction in prostate cancer cells, only HSP27 showed a statistically significant reaction according to the Gleason score and pathological stages; they also reported that the expression level of HSP27 is a useful predictive factor that predicts biochemical recurrence in prostate cancer. Rocchi et al [5] found that the increase in HSP27 protected the cells in the process of CRPC development after hormone treatment in prostate cancer; they also found that HSP27 played a role as the apoptosis controller. Teimourian et al [14] proved that the viability significantly decreased after 72 hours' irradiation in prostate cancer cell lines that decreased HSP27 expression compared to prostate cancer cell lines it unchanged in HSP27 expression. The development of CRPC has been linked to the expression of anti-apoptotic HSP27 protein blocking the induction of programmed cell death, which is triggered by a variety of conventional and novel chemotherapeutic regimens [5]. Recent data links increased HSP27 with hormone resistance and poor outcomes in prostate cancer. Increased levels of HSP27 after androgen withdrawal provide a cytoprotective role during development of androgen independence. PC-3 expresses a higher level of HSP27 mRNA in vitro and in vivo. Phosphorothioate HSP27 antisense oligonucleotides (ASOs) and small interference RNA potently inhibit HSP27 expression, with increased caspase-3 cleavage and PC-3 apoptosis and decreased PC-3 growth. Thus ASO-induced silencing enhanced apoptosis of cancer and delayed tumor progression. We investigated whether apoptosis in prostate cancer cells and Gleason's score of prostate cancer cells are associated with expression of HSP27 [15]. In the present study, it was inferred that HSP27 mRNA expression was associated with the malignancy level of prostate cancer, and a high doxazosin concentration was associated with higher expression of HSP27. In the immunostaining of HSP27, treatment with 25 µM doxazosin showed more intense staining than treatment with 10 µM.
Quinazoline-based 1-blockers, such as doxazosin and terazosin, have shown anti-tumor efficacy by induction of apoptosis in prostate cancer cells though 1-adrenoceptor-independent mechanisms [16]. It has been reported that doxazosin and terazosin suppress the invasion and migration of prostate cancer and endothelial cells in order to reduce adhesion potential with extracellular matrix components and to induce apoptosis in prostate cancer cells. HSP27 down-regulation lowered the apoptotic threshold in both PC-3 and LNCaP cells. That control siRNA did not show significant induction of apoptosis, whereas the HSP27 siRNA compound induced programmed cell death, supports the notion of specificity for the HSP27 siRNA treatment tested [17].
Cell death induced by HSP27 siRNA is greater in the androgen receptor-negative cell line PC-3 than in the androgen receptor-positive cell line LNCaP [18]. One possible explanation is that the androgen receptor is more important for the balance of growth/apoptosis, while other survival factors, like HSP27, become more relevant in androgen receptor-negative PC-3 cells [19,20].
This study just suggests the possibility of treatment of prostate cancer through suppression of HSP27 and doxazosin's effect. In the future, we need to clarify doxazosin's direct effect on apoptosis and DNA damage, acting via a pathway transduced by doxazosin binding to a membrane receptor.
It was verified that HSP27 expression patterns specific to individual prostate cancer cell lines increased depending on the malignancy levels of cancer cells. It was also possible to verify this tendency by immunofluorescence staining. These results showed that HSP27 expression was related to the prostate cancer malignancy level. Additionally, the higher the treatment of PC-3 with doxazosin, the higher the HSP27 expression was; through this, it was verified that stress was related to prostate cancer apoptosis.
Figures and Tables
 | Fig. 1HSP27 reverse transcription polymerase chain reaction results according to the cell type (RWPE-1, LNCaP, PC-3, and TSU-Pr1). The density of HSP27 mRNA expression was shown to be higher than the low grade malignant cell line. Values are mean±standard error of mean. HSP27: heat shock protein 27, GAPDH: glyceraldehyde-3-phosphate dehydrogenase. ap<0.05 vs. control. |
 | Fig. 2Heat shock protein 27 immunofluorescence staining according to the cell type (RWPE-1, LNCaP, PC-3, and TSU-Pr1). The staining for TSU-Pr1 was more intense than that of the other cell lines. The intensity of staining was RWPE-1<LNCaP<PC-3<TSU-Pr1. ×200; Fluoview confocal laser scanning microscope (Olympus Optical Co., Ltd., Tokyo, Japan). |
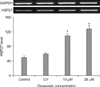 | Fig. 3HSP27 gene expression patterns according to the doxazosin treatment concentration. The HSP27 gene expression with 25 µM of doxazosin treatment was higher than that with 10 µM (Control, CV, 10 µM, 25 µM). Values are mean±standard error of mean. HSP27: heat shock protein 27, GAPDH: glyceraldehyde-3-phosphate dehydrogenase. ap<0.05 vs. control. |
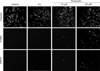 | Fig. 4HSP27 immunofluorescence staining of PC-3 with doxazosin treatment (Control, CV, 10 µM, 25 µM). ×200; Fluoview confocal laser scanning microscope (Olympus Optical Co., Ltd., Tokyo, Japan). CV: control vehicle, HSP27: heat shock protein 27, TUNEL: terminal transferase-mediated biotinylated 16-desoxy-uridine triphosphate nick-end labeling. |
References
1. Kim JM, Kim HM, Jung BY, Park EC, Cho WH, Lee SG. The association between cancer incidence and family income: analysis of Korean National Health Insurance cancer registration data. Asian Pac J Cancer Prev. 2012; 13:1371–1376.

2. Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005; 65:11083–11093.

3. Hessenkemper W, Baniahmad A. Targeting heat shock proteins in prostate cancer. Curr Med Chem. 2013; 20:2731–2740.

4. Atkins D, Lichtenfels R, Seliger B. Heat shock proteins in renal cell carcinomas. Contrib Nephrol. 2005; 148:35–56.

5. Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, Fazli L, et al. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004; 64:6595–6602.

6. Youm YH, Kim S, Bahk YY, Yoo TK. Proteomic analysis of androgen-independent growth in low and high passage human LNCaP prostatic adenocarcinoma cells. BMB Rep. 2008; 41:722–727.

7. Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000; 60:4550–4555.
8. Giardinà D, Martarelli D, Sagratini G, Angeli P, Ballinari D, Gulini U, et al. Doxazosin-related alpha1-adrenoceptor antagonists with prostate antitumor activity. J Med Chem. 2009; 52:4951–4954.
9. Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001; 286:433–442.

10. Sarto C, Valsecchi C, Magni F, Tremolada L, Arizzi C, Cordani N, et al. Expression of heat shock protein 27 in human renal cell carcinoma. Proteomics. 2004; 4:2252–2260.

11. Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr. 2001; 9:195–201.

12. Erkizan O, Kirkali G, Yörükoğlu K, Kirkali Z. Significance of heat shock protein-27 expression in patients with renal cell carcinoma. Urology. 2004; 64:474–478.

13. Miyake H, Muramaki M, Kurahashi T, Takenaka A, Fujisawa M. Expression of potential molecular markers in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol Oncol. 2010; 28:145–151.

14. Teimourian S, Jalal R, Sohrabpour M, Goliaei B. Down-regulation of Hsp27 radiosensitizes human prostate cancer cells. Int J Urol. 2006; 13:1221–1225.

15. Dowling AJ, Tannock IF. Systemic treatment for prostate cancer. Cancer Treat Rev. 1998; 24:283–301.

16. Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996; 28:251–265.

17. Landry J, Chrétien P, Laszlo A, Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991; 147:93–101.

18. Huot J, Lambert H, Lavoie JN, Guimond A, Houle F, Landry J. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur J Biochem. 1995; 227:416–427.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download