Abstract
The evolution of the artificial urinary sphincter has affected the current surgical options for urinary incontinence. With its unique features, the artificial urinary sphincter (AUS) has been an attractive option for the treatment of urinary incontinence regardless of gender. The current paper discusses the indications, contraindications, types of devices, surgical approaches, outcomes, and complications of the AUS in the treatment of both male and female urinary incontinence. A PubMed review of the available literature was performed and articles reporting implantation of artificial urinary sphincters for urinary incontinence in both male and female patients were evaluated. There was a comparable satisfactory continence rate after the implantation of an AUS (59~97% in males vs. 60~92% in females). In comparison, there were some differences in the indications, contraindications, surgical approaches, outcomes, and complications of the AUS implanted for urinary incontinence in male and female patients. AUS implantation is a safe and effective surgical option for the treatment of urinary incontinence of various etiologies. Continuous evolution of the device has made it an attractive option for the treatment of both male and female urinary incontinence.
Go to : 
When Trost and Elliott described the first artificial sphincter in 1947, the external model was largely different from the current model, being controlled by a detachable pump held in the pocket.1 Subsequent modification of the model by Trost and Elliott1 led to the current version of the AUS first implanted by Petero and Diokno2 That model, the AS721, despite being effective, was associated with high rate of complications, failures, and subsequent revision and explantation. To reduce these problems and to further improve the efficacy, newer versions of the American Medical Systems (AMS) artificial urinary sphincter (AUS) were developed until 1983, when the AMS800, the fifth generation of the AMS AUS, was introduced and widely regarded as the AUS of choice for almost 29 years thereafter. Recently, the new Flow Secure AUS was invented, not only to improve the efficacy of the most commonly used AMS800 but also to reduce the complications rate namely urethral atrophy, erosion, and infection, which most of the time required subsequent surgical revision or device explantation.
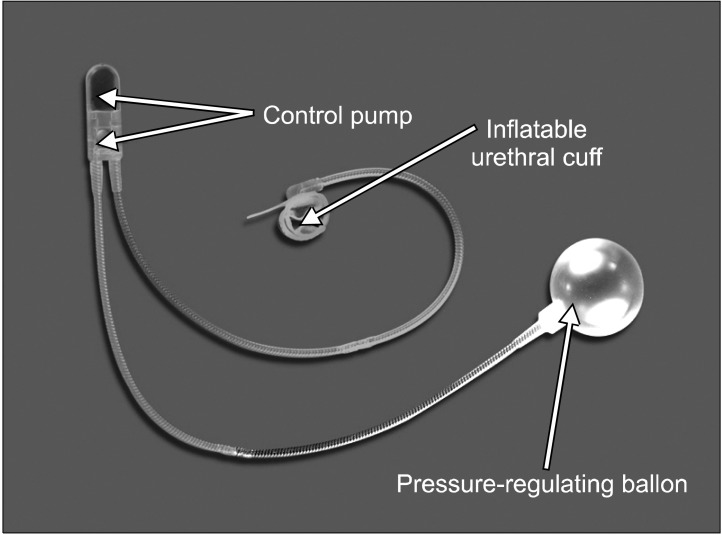
The AUS consists of three components: the inflatable urethral cuff, the pressure-regulating balloon or reservoir, and the control pump, all of which are connected with tubing systems (Fig. 1). The entire system is filled with fluid, and the device functions using principles of hydraulics. The occlusive cuff provides pressure to maintain continence. The pressure-regulating balloon reservoir regulates the pressure inside the system. During voiding, the fluid inside the cuff is released. This leads to reduction of pressure inside the cuff and allows for the flow of urine. There are various cuff sizes and balloon pressures to adapt to various patient requirements.
The AUS is a complex device. A number of improvements have been made to the device to ease implantation and subsequently reduce the complication, reoperation, and explantation rates. Despite that, the reoperation rate for AUS implantation remains high. There are many medical reasons for the high reoperation, rate but the main reason is still the surgeon's experience. Sandhu et al3 concluded that, in contrast to a surgeon's typical experience, the learning curve for AUS surgery appears to be very long and without an obvious plateau suggesting a considerable burden of avoidable reoperations.
Go to : 
The AUS was initially implanted primarily in males with urinary incontinence mainly due to radical prostatectomy. The incidence of incontinence after open perineal or retropubic prostatectomy ranges between 0.5 and 40%.4 There is no significant difference in the incidence of post-radical prostatectomy incontinence depending on whether it is done laparoscopically, robotically, or by open operation.5 There is also no difference in the incidence rate based on whether the open radical prostatectomy is performed via a perineal or retropubic approach.5 Currently, there are transitions of indications for implantation of AUS, which include male and female urinary incontinence for various reasons. While post-prostatectomy incontinence remains the main indication for artificial sphincter implantation, there is an increasing trend toward implanting an artificial sphincter for other indications including female urinary incontinence.
AUS implantation should be considered in incontinence secondary to low urethral pressure. Ratan et al6 reported that 90% of AUS implantations were performed in post-prostatectomy patients. The AUS has been considered to be the gold standard for the treatment of post-prostatectomy urinary incontinence. Nowadays, more and more AUS devices have been implanted in incontinence for other causes. The AUS is also indicated in male urinary incontinence secondary to surgery related to benign prostate conditions such as transurethral resection of the prostate (TURP), photoselective vaporization of the prostate (PVP), or holmium laser enucleation of the prostate (HOLEP) and in incontinence after radiation therapy to the prostate. TURP has been associated with a 1~5% risk of incontinence.7-10 The incidence of post-PVP incontinence is 0~6.5%.11-14 In HOLEP, the reported incidence of incontinence after surgery is 2.2~7.1%.9,11,15-17 The reported risk of incontinence in males post-radiotherapy is 0~18.8%.5 Neuropathic bladder dysfunction with incontinence secondary to intrinsic sphincter deficiency is also an important indication for implantation of an AUS regardless of age and gender.6
To date, a definitive role for the AUS in adult female urinary incontinence has not been found. Despite that, more and more patients with severe stress urinary incontinence secondary to intrinsic sphincter deficiency have been offered AUS implantation. However, AUS has usually been performed as a last resort after other forms of treatment have failed. Congenital causes of incontinence constitute one of the indications for implantation of an AUS for urinary incontinence in females.18-20 The other indication for implantation of an AUS in females is incontinence secondary to neurological diseases.2,18-21
Orthotopic neobladder has become the standard choice of urinary diversion for cystectomy patients due to the range of diseases, mainly carcinoma of the bladder. Due to the increasing numbers of orthotopic neobladder created, there are also increasing numbers of patients with urinary incontinence, which is one of its known complications. The incidence of incontinence after orthotopic neobladder creation range between 85 and 100% during the day and 55 to 100% during the night.5 Since the first study reported by O'Connor et al22 regarding the success of the AUS in patients with orthotopic neobladder. there are increasing numbers of AUS devices implanted in patients post-cystectomy with orthotopic neobladder. Infection remains a matter of concern as the orthotopic neobladder is normally colonized with microorganisms but O'Connor et al22 has reported no increased risk of infection for an AUS implanted in orthotopic neobladder cases.
Go to : 
AUS implantation in males should never be performed until 6~12 months after the resulting initial event.1 Within this period of time urinary incontinence may improve by itself without intervention. On the other hand, improvement is considered unlikely after this period of time has elapsed. Candidates for AUS implantation should have normal bladder capacity and compliance without any intraurethral or intravesical pathology. Furthermore, such patients should have sufficient physical and mental capacity to deal with the AUS device.
As complications, surgical revisions, and device explantations remain the main concern in AUS implantation, certain measures should be undertaken prior to and during the implantation. The patient should have sterile mid-stream urine before the operation. While in the operation theatre, the patient should undergo a 10 minutes betadine scrub and have the pubic hair shaved. During the operation, waterproof drapes and gowns should be used with the surgeon and assistants wearing double gloves. Antibiotic irrigation should be done during the operation. Hematoma formation should be avoided and post-operative antibiotics should be continued. There should be minimal traffic during the operation.6
Go to : 
The AUS is contraindicated in patients with repetitive urinary infection, urethral diverticula at the expected implante site, in complex, unstable, or recurrent urethral stricture diseases, in small capacity and/or non-compliant bladder prior to definitive treatment, in irreversibly obstructed urinary tract or in patients with a lack of physical or mental dexterity to manipulate the pump.23-27 It is relatively contraindicated in patients with high-grade vesicoureteric reflux, with recurrent intravesical or intraurethral diseases such as stones or tumors, with bladder neck contracture prior to treatment, and with detrusor overactivity.23,25,28
Radiotherapy-induced incontinence is considered by some scholars to be a contraindication for implantation of the AUS in female patients.18 Thomas et al18 reported a 100% failure rate and considered a history of pelvic radiotherapy as an absolute contraindication to placement of the AUS in women. Vayleux et al29 in their series of women with AUS, confirmed that radiation therapy increases the risk of complications, especially urethral atrophy, cuff erosion, and infection, with subsequent increase in the risk of device failure and revision surgery. However, radiotherapy is not considered to be a contraindication for placement of the AUS in men,30 as the male urethra is longer and the AUS can be implanted around the bulbar urethra. Walsh et al30 reported a similar long-term outcome of AUS in male patients after prostatectomy with or without radiation therapy despite a higher rate of complications in the former. Kim et al31 and Gomha and Boone32 reported that radiation therapy was not associated with an increased risk of AUS complications.
Go to : 
Currently, the most widely available device is the AMS800 (Amedican Medical System Holdings Inc., Minnetonka, MN, USA) with new devices being released to improve the outcome (Fig. 1).
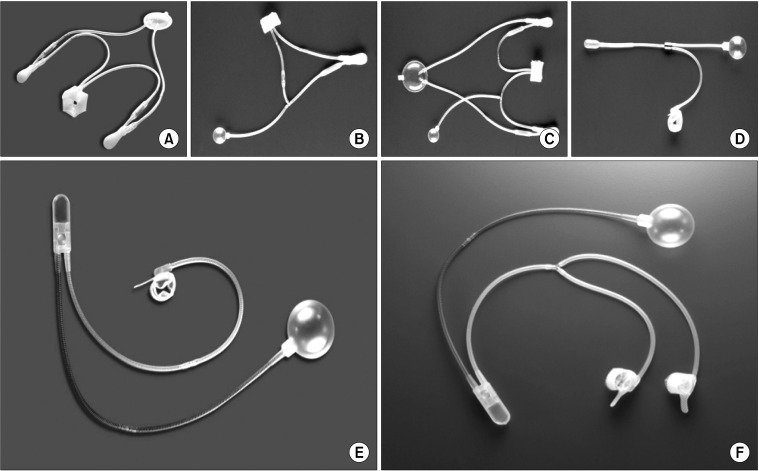
Petero and Diokno2 created the first AMS device, which was the AS721 (Fig. 2A). Then, the new AMS series was developed, which included the AS742 (Fig. 2B), AS761 (Fig. 2C), AS791/792 (Fig. 2D), and the current most widely used AMS800 (Fig. 2E, 2F). The AS 721, which was the first of the AMS series created, was composed of 4 components connected to each other with valved tubes: the inflatable cuff, the pressure-regulating balloon reservoir, the activating pump, and the deactivating pump. It has the disadvantages of having large numbers of components and tubes, which necessitate extensive dissection with subsequent higher risk of complications and failures. Since then, a great deal of effort has been put into improving the device. The AMS800 is the 5th generation of the AMS series and is now regarded as the gold standard for AUS implantation. The difference between the AMS800 and the AS721 is that the AMS800 incorporates the activating and deactivating pumps into one pump and has kink resistant tubing. Additional refinements include surface treatment, change of shape, such as a narrower back cuff, and color-coded tubes.
Despite all the improvements to the AMS series, the complications and subsequent revision surgery rates are still considered high and further improvement in the outcomes is needed. Therefore, new non-AMS devices have emerged. Vakalopoulos et al33 have come out with the new Flow Secure device, which has the advantage of being a one-piece device. The Flow Secure device, in contrast to the AMS device, is filled with normal saline instead of contrast and cannot be monitored with normal radiological imaging. For that reason, ultrasound is needed for monitoring. In addition, it has two reservoirs among which the stress relief balloon reservoir provides additional pressure to the cuff during increased intraabdominal pressure, thus improving urinary continence.33 The other new device, which has just recently been made available on the market, is the Periurethral Constrictor (Silimed, Rio de Janeiro, Brazil).33 This device is mainly used in pediatric patients. It is a one-piece device with two segments or compartments. It works hydraulically by the injection of sterile solution through the self-sealing valve in order to promote a static occlusive pressure on the cuff. It has the advantages of being simple and cheap. Zephyr surgical implants, a Swiss-French company, has produced a new artificial sphincter device, the ZSI375, designed by Gomez Llorens.33 This device is indicated in stress urinary incontinence secondary to intrinsic sphincter deficiency. It is a one-piece model with two compartments separated by a piston: the hydraulic circuit and the compensation pouch circuit. In addition to the pressure in the hydraulic circuit, the deflated cuff also compresses the urethra, which improves the efficacy and the continence. The ZSI375 has the advantages of increasing the issued pressure and allowing for cuff adjustability. Another new device is the Tape Mechanical Occlusive Device (GU Urological, Minneapolis, MN, USA).33 This is a one-piece device that is manually controlled by the patient through its on and off button. It has two tapes, both of which are connected to the control mechanism: the occlusive tape and the conduit tape.
Go to : 
When the AUS was initially introduced, it was mainly implanted via the combined perineal and abdominal approach for male urinary incontinence. As the indications for implantation of the AUS broaden, and as females and children are included, the AUS implantation method now also varies more. The evolution of minimally invasive surgery has had an influence, as the AUS is now being laparoscopically implanted.34-37 Thus, AUS implantation can be classified into an open or laparoscopic approach.
1) The combined perineal and abdominal approach is considered the traditional approach for implantation of the AUS in males. The perineal incision is for the implantation of the cuff while the abdominal incision is for the balloon reservoir.
2) The trans-scrotal approach has the advantage of having only a single incision, thus reducing the operation time and shortening the hospital stay. Wilson et al38 has proposed that a single scrotal incision was easier and faster than the traditional two-incision technique. In a patient who has both the problems of incontinence and impotence, a single trans-scrotal incision provides access for the implantation of both the AUS and a penile implant, a device called AMS1500. However, despite the advantages, many experts still believed that the outcome of the trans-scrotal approach is inferior compared to the traditional combined perineal and abdominal approach. It has a lower complete dry rate (27.4% compared to 44.1% in the perineal approach).39
3) The abdominal approach is mainly used when the intended site for implantation of the AUS is at the bladder neck, especially in females. It is done via the suprapubic approach. It can be challenging, as the AUS for female urinary incontinence is usually a last resort after failure of previous surgeries.
4) The combined abdominal and transvaginal approach can be done for implantation of the AUS in females. Popularized by Appell40 in 1988, this approach mirrors the combined perineal and abdominal approach for males whereby the transvaginal incision is for the insertion of the cuff while the abdominal incision is for the placement of the balloon reservoir. He reported an incredible 100% success rate with this approach.
2. The laparoscopic method of AUS implantation35-37 is a minimally invasive approach for implantation of the AUS trans-abdominally. It is mainly done for female urinary incontinence. Unlike the open approach, it has the advantage of approaching the site of interest via a virgin area. However, it has a steeper learning curve and requires expertise in laparoscopic skills.
Go to : 
Many studies have assessed the outcomes of AUS implantation. However, interpretation and direct comparison are difficult due to variability of definitions, surgical techniques, and selection of patients or research populations.
The overall continence rate after implantation of an AUS is 73% and the improved continence rate is 88% in the published literature.41 Venn et al19 reported an 84% continence rate after AUS implantation in both male and female patients at 10 years of follow up. The evolution of new devices has improved the long-term outcomes and reduced the complication rates.
The outcome of AUS implantation differ somewhat in males and females as shown in Table 1. In 1983, Barrett and Furlow49 reported a 74% continence rate in AUS implantation for post-prostatectomy incontinence with improvement to 93% when the patients with radiotherapy were excluded. However, this study used an old version of the AUS (AS791). Brito et al,50 with their double cuff technique, improved the continence rate to over 95%. O'Connor et al,43 however, reported no difference in outcome but higher revision with the double cuff technique. Singh and Thomas42 defined a successful outcome as a 'social continence rate' (complete continence or minor stress leakage) and reported a 96% continence rate at a mean of 41 months of follow up. Litwiller51 raised the issue of patient satisfaction and exclusively added patient satisfaction in his study. He reported a 97% social continence rate (20% complete continence, 55% a few drops of leakage, 22% less than a teaspoonful of urine/day) at a median follow up of 23.4 months.33 Despite the fact that only 20% achieved complete continence after surgery, 90% of the patients were satisfied with the outcome.51 This shows that patient satisfaction does not necessarily require the patients to be completely dry but rather to experience an improvement in urinary continence. Gousse et al44 took the same approach with Litwiller51 in their study with a mean follow up of 7 years. In contrast to the result reported by Litwiller51, Gousse et al44 reported only 77% patient satisfaction (58% very satisfied, 19% satisfied, and 23% unsatisfied) with the outcome of surgery. The difference between the study by Gousse et al44 and Litwiller et al51 is the duration of the study. Gousse et al44 had a longer study duration. This demonstrates that the longer the duration of AUS in situ, the greater the risk of deterioration, failure, and complications and the lower the patient satisfaction rate.
In comparison to the AUS in males, fewer studies have addressed female urinary incontinence. An earlier review by Venn et al19 showed that outcomes for females were less favorable than for males. However, a later study by Petero and Diokno2 reported no difference in outcomes of AUS in males and females. Costa et al48 has done one of the largest studies concerning AUS in female urinary incontinence of various etiologies. He reported 88.7% and 81.8% continence rates in non-neurogenic and neurogenic causes of urinary incontinence, respectively. Furthermore, the social continence rate was reported as high as 96.4% and 90.9%, respectively.48 On the other hand, Thomas et al,18 who reported an 81% continence rate, had the longest follow-up period of any study we reviewed. Donovan et al21 reported a success rate of 61% from 31 patients, mostly with neuropathic bladders, while Light and Scott52 reported a success of 89% from 39 patients with stress incontinence. Webster et al53 reported a good continence rate of 92% in patients treated with AUS implantation primarily for ISD. However, the continence rate decreased to 60% when the AUS was placed in multiply operated patients,46 which is commonly encountered, as the AUS is normally implanted in females as a last resort after the failure of other methods.
One of the most concerning problems of AUS is the high rate of revision surgery. It has been reported that the overall reoperation rate for AUS implantation is 28~35%.19,42,54 Venn et al19 reported a 26% reoperation rate, which included both genders. This may be due to technical errors, bladder dysfunction, and mechanical and non-mechanical errors. Once these complications occur, investigations by cystoscopy, and urodynamic and radiological imaging must be performed.
Singh and Thomas42 reported a 36% reoperation rate in their study of post-prostatectomy incontinence. However, following a number of technical improvements of the device, the rate of revision surgery has decreased. Trost and Elliott1 at the Mayo Clinic reported a significant decrease in revision surgery from 42% to 17% following the improvement of the device.42 In males, the explantation rate has been reported to be 16~20.3%1.
In females, the reoperation rate for AUS ranges from 0 to 80%.29 Furthermore, the explantation rate was in the range of 20~50% in the literature.29 However, Verlaux et al18 had a lower explantation rate of 7%, which was comparable to the study by Costa et al.48 Venn et al19 stratified their patients by neurogenic and non-neurogenic patients and found that 50% of the female patients with neurogenic causes of incontinence had their AUS explanted in comparison to 30% of male patients. This may be because the AUS cuff is inserted around the female bladder neck, which is a more difficult surgery in comparison to placement of the AUS cuff around the male bulbous urethra.19 Furthermore, the female urethra is thinner than the male bladder neck and AUS implantation is normally performed in females after previous failed attempts at surgery.19
Go to : 
AUS implantation is not without untoward complications. Kim et al31 reported an overall complication rate of 37%. Common complications include mechanical failure, urethral erosion, infection, and atrophy leading to recurrent incontinence and subsequently device revision or explantation.
The overall rate of mechanical device failure is 14%.41 This incidence was reduced to 7.6% after the introduction of the narrow-backed cuff device.42,46 Mechanical device failure is estimated to occur in 7.6~21% of male patients.6,47 Most of the time, it is due to leakage of fluid from the hydraulic system, either from the cuff, the balloon, or the tubing. Total or partial device reimplantation is most likely needed after device failure. Vayleux et al29 reported a 15.3% incidence of device failure in a series of female patients. They identified several risk factors of device failure and subsequent explantation of AUS, including history of radiotherapy, age >70, and history of previous surgery. However, the body mass index, number of previous operations, parity number or positive Marshall Bonney test were not found to be risk factors in their analysis of the series.
Infection is the most devastating complication and should be avoided by all means. Hajivassiliou41 reported a 4.5% incidence of device infection. The incidence of device infection in adult males is 1~14%.1 Costa et al20 reported a 4.8% risk of device infection in a series of female patients. Infection necessitates device removal and delayed reimplantation.
Urethral erosion after AUS implantation is a disastrous complication. It can happen early post-operatively or later after convalescence. An overall incidence of delayed erosion of up to 15% for both genders has been reported.46 In males, the incidence has been reported to be 4~10%.1 In females, Costa et al20 reported a 8.1% incidence of erosion, which includes vagina, labia majora, urethral, and bladder erosion. Once erosion occurs, device removal and delayed reimplantation are almost inevitable. Early urethral erosion happens most commonly due to infection or a technical error during implantation, while delayed urethral erosion occurs due to urethral damage secondary to cuff pressure and improper catheterization.
Urethral atrophy is not infrequent. In males, an incidence rate of 4~10% has been reported.1 It may result secondary to chronic compression of the urethra. It commonly presents with recurrent incontinence after a period of continence. However, in females, the incidence of urethral atrophy after AUS implantation is not well documented in the literature.
Go to : 
The artificial urinary sphincter is safe and effective in management of urinary incontinence in both males and females. While being a gold standard in treating male urinary incontinence, it is also slowly gaining acceptance for female urinary incontinence. Despite being effective with a high satisfaction rate, the pitfall of the AUS remains the high complication, reoperation and explantation rates. Evolution in the design of the device and surgical technique has reduced but not eliminated these problems. Continuous improvement should be done for AUS to make it the method of choice in urinary incontinence of various etiologies.
Go to : 
References
1. Trost L, Elliott DS. Male stress urinary incontinence: a review of surgical treatment options and outcomes. Adv Urol. 2012; 2012:287489. PMID: 22649446.

2. Petero VG Jr, Diokno AC. Comparison of the long-term outcomes between incontinent men and women treated with artificial urinary sphincter. J Urol. 2006; 175:605–609. PMID: 16407005.

3. Sandhu JS, Maschino AC, Vickers AJ. The surgical learning curve for artificial urinary sphincter procedures compared to typical surgeon experience. Eur Urol. 2011; 60:1285–1290. PMID: 21665357.

5. Herschorn S, Bruschini H, Comiter C, Grise P, Hanus T, Kirschner-Hermanns R, et al. Committee of the International Consultation on Incontinence. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010; 29:179–190. PMID: 20025026.

6. Ratan HL, Summerton DJ, Wilson SK, Terry TR. Development and current status of the AMS 800 artificial urinary sphincter. EAU-EBU Update Ser. 2006; 4:117–128.

7. Buckley BS, Lapitan MC. Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children--current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010; 76:265–270. PMID: 20541241.

8. Khoury S, Cockett A, Aso Y, Chatelain C, Andersson L, Abrams P, et al. International Consultation on Urological Diseases: a decade of progress. Prostate. 2000; 45:194–199. PMID: 11027419.

9. Cho MC, Park JH, Jeong MS, Yi JS, Ku JH, Oh SJ, et al. Predictor of de novo urinary incontinence following holmium laser enucleation of the prostate. Neurourol Urodyn. 2011; 30:1343–1349. PMID: 21538499.

10. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003; 170:530–547. PMID: 12853821.
11. Son H, Song SH, Paick JS. Current laser treatments for benign prostatic hyperplasia. Korean J Urol. 2010; 51:737–744. PMID: 21165192.

12. Te AE, Malloy TR, Stein BS, Ulchaker JC, Nseyo UO, Hai MA, et al. Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: 12-month results from the first United States multicenter prospective trial. J Urol. 2004; 172:1404–1408. PMID: 15371855.

13. Spaliviero M, Araki M, Culkin DJ, Wong C. Incidence, management, and prevention of perioperative complications of GreenLight HPS laser photoselective vaporization prostatectomy: experience in the first 70 patients. J Endourol. 2009; 23:495–502. PMID: 19265468.
14. Park JH, Son H, Paick JS. Comparative analysis of the efficacy and safety of photoselective vaporization of the prostate for treatment of benign prostatic hyperplasia according to prostate size. Korean J Urol. 2010; 51:115–121. PMID: 20414424.

15. Elzayat EA, Habib EI, Elhilali MM. Holmium laser enucleation of the prostate: a size-independent new "gold standard". Urology. 2005; 66(5 Suppl):108–113. PMID: 16194716.

16. Elzayat EA, Elhilali MM. Holmium laser enucleation of the prostate (HoLEP): the endourologic alternative to open prostatectomy. Eur Urol. 2006; 49:87–91. PMID: 16314033.

17. Vavassori I, Hurle R, Vismara A, Manzetti A, Valenti S. Holmium laser enucleation of the prostate combined with mechanical morcellation: two years of experience with 196 patients. J Endourol. 2004; 18:109–112. PMID: 15006063.

18. Thomas K, Venn SN, Mundy AR. Outcome of the artificial urinary sphincter in female patients. J Urol. 2002; 167:1720–1722. PMID: 11912395.

19. Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol. 2000; 164:702–706. PMID: 10953129.

20. Costa P, Poinas G, Ben Naoum K, Bouzoubaa K, Wagner L, Soustelle L, et al. Long-term results of artificial urinary sphincter for women with type III stress urinary incontinence. Eur Urol. 2013; 63:753–758. PMID: 22445222.

21. Donovan MG, Barrett DM, Furlow WL. Use of the artificial urinary sphincter in the management of severe incontinence in females. Surg Gynecol Obstet. 1985; 161:17–20. PMID: 4012538.
22. O'Connor RC, Kuznetsov DD, Patel RV, Galocy RM, Steinberg GD, Bales GT. Artificial urinary sphincter placement in men after cystectomy with orthotopic ileal neobladder: continence, complications, and quality of life. Urology. 2002; 59:542–545. PMID: 11927310.
23. Smith JJ, Barrett DM. Glenn JF, Keane TE, Brendler CB, Gormley EA, Jordan GH, Kavoussi LR, editors. Artificial urinary sphincter for incontinence. Glenn's urologic surgery. 2004. 6th ed. Philadelphia: Williams & Wilkins;p. 414–421.
24. Wessells H, Peterson AC. Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Surgical procedures for sphincteric incontinence in the male: the artificial genitourinary sphincter and perineal sling procedures. Campbell's urology. 2012. 10th ed. Philadelphia: Saunders;p. 2290–2305.
26. Elliott DS, Barrett DM. The artificial urinary sphincter in the female: indications for use, surgical approach and results. Int Urogynecol J Pelvic Floor Dysfunct. 1998; 9:409–415. PMID: 9891964.

27. Schiavini JL, Damião R, de Resende Júnior JA, Dornas MC, Cruz Lima da Costa DS, Barros CB. Treatment of post-prostate surgery urinary incontinence with the periurethral constrictor: a retrospective analysis. Urology. 2010; 75:1488–1492. PMID: 20399484.

28. Leibovich BC, Barrett DM. Use of the artificial urinary sphincter in men and women. World J Urol. 1997; 15:316–319. PMID: 9372584.

29. Vayleux B, Rigaud J, Luyckx F, Karam G, Glémain P, Bouchot O, et al. Female urinary incontinence and artificial urinary sphincter: study of efficacy and risk factors for failure and complications. Eur Urol. 2011; 59:1048–1053. PMID: 21420781.

30. Walsh IK, Williams SG, Mahendra V, Nambirajan T, Stone AR. Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. BJU Int. 2002; 89:364–368. PMID: 11872025.

31. Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008; 179:1912–1916. PMID: 18353376.

32. Gomha MA, Boone TB. Artificial urinary sphincter for post-prostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J Urol. 2002; 167:591–596. PMID: 11792924.

33. Vakalopoulos I, Kampantais S, Laskaridis L, Chachopoulos V, Koptsis M, Toutziaris C. New artificial urinary sphincter devices in the treatment of male iatrogenic incontinence. Adv Urol. 2012; 2012:439372. PMID: 22567002.

34. Gamé X, Bram R, Abu Anz S, Doumerc N, Guillotreau J, Malavaud B, et al. Laparoscopic insertion of artificial periprostatic urinary sphincter. Urology. 2009; 73:442.e1–442.e3. PMID: 18533234.

35. Rouprêt M, Misraï V, Vaessen C, Cardot V, Cour F, Richard F, et al. Laparoscopic approach for artificial urinary sphincter implantation in women with intrinsic sphincter deficiency incontinence: a single-centre preliminary experience. Eur Urol. 2010; 57:499–504. PMID: 19346059.

36. Mandron E, Bryckaert PE, Papatsoris AG. Laparoscopic artificial urinary sphincter implantation for female genuine stress urinary incontinence: technique and 4-year experience in 25 patients. BJU Int. 2010; 106:1194–1198. PMID: 20132197.

37. Ngninkeu BN, van Heugen G, di Gregorio M, Debie B, Evans A. Laparoscopic artificial urinary sphincter in women for type III incontinence: preliminary results. Eur Urol. 2005; 47:793–797. PMID: 15925075.

38. Wilson S, Delk J 2nd, Henry GD, Siegel AL. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003; 169:261–264. PMID: 12478150.

39. Henry GD, Graham SM, Cornell RJ, Cleves MA, Simmons CJ, Vakalopoulos I, et al. A multicenter study on the perineal versus penoscrotal approach for implantation of an artificial urinary sphincter: cuff size and control of male stress urinary incontinence. J Urol. 2009; 182:2404–2409. PMID: 19762042.

40. Appell RA. Techniques and results in the implantation of the artificial urinary sphincter in women with type III stress urinary incontinence by a vaginal approach. Neurourol Urodyn. 1988; 7:613–619.

41. Hajivassiliou CA. A review of the complications and results of implantation of the AMS artificial urinary sphincter. Eur Urol. 1999; 35:36–44. PMID: 9933793.

42. Singh G, Thomas DG. Artificial urinary sphincter for post-prostatectomy incontinence. Br J Urol. 1996; 77:248–251. PMID: 8800893.

43. O'Connor RC, Lyon MB, Guralnick ML, Bales GT. Long-term follow-up of single versus double cuff artificial urinary sphincter insertion for the treatment of severe postprostatectomy stress urinary incontinence. Urology. 2008; 71:90–93. PMID: 18242372.
44. Gousse AE, Madjar S, Lambert MM, Fishman IJ. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol. 2001; 166:1755–1758. PMID: 11586217.

45. Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998; 159:1206–1208. PMID: 9507835.

46. Hussain M, Greenwell TJ, Venn SN, Mundy AR. The current role of the artificial urinary sphincter for the treatment of urinary incontinence. J Urol. 2005; 174:418–424. PMID: 16006857.

47. Webster GD, Sherman ND. Management of male incontinence following artificial urinary sphincter failure. Curr Opin Urol. 2005; 15:386–390. PMID: 16205488.

48. Costa P, Mottet N, Rabut B, Thuret R, Ben Naoum K, Wagner L. The use of an artificial urinary sphincter in women with type III incontinence and a negative Marshall test. J Urol. 2001; 165:1172–1176. PMID: 11257664.

49. Barrett DM, Furlow WL. Radical prostatectomy incontinence and the AS791 artificial urinary sphincter. J Urol. 1983; 129:528–530. PMID: 6834538.

50. Brito CG, Mulcahy JJ, Mitchell ME, Adams MC. Use of a double cuff AMS800 urinary sphincter for severe stress incontinence. J Urol. 1993; 149:283–285. PMID: 8426402.

51. Litwiller SE, Kim KB, Fone PD, White RW, Stone AR. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol. 1996; 156:1975–1980. PMID: 8911369.

52. Light JK, Scott FB. Management of urinary incontinence in women with the artificial urinary sphincter. J Urol. 1985; 134:476–478. PMID: 4032543.

53. Webster GD, Perez LM, Khoury JM, Timmons SL. Management of type III stress urinary incontinence using artificial urinary sphincter. Urology. 1992; 39:499–503. PMID: 1615594.

54. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of followup. J Urol. 1997; 158:435–439. PMID: 9224318.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download