Abstract
The presence of ectopic prostate tissue in the bladder is common, but the involvement of the bladder dome has rarely been reported. This case report describes a 72-year-old man who presented with gross painless hematuria. Cystoscopy revealed a smooth sessile mass at the dome region of the bladder. A complete transurethral resection of the mass was performed. Histopathological examination of the mass revealed the presence of benign ectopic prostatic tissue.
Ectopic prostatic tissue is relatively uncommon and has been underreported.1 It is most commonly encountered in the lower male genitourinary tract, but is also found in pericolic fat and the female genitourinary tract, anal canal, retroperitoneum, and spleen.2 Ectopic prostate tissue in the bladder usually involves the midline in the form of the vestigial remains of embryonic prostatic elements.2 Only 4 cases of this lesion have been reported previously in the English medical literature.2-4 This report presents an unusual case of ectopic prostate tissue at the bladder dome confirmed with cystoscopy, and this report also reviews the relevant literature.
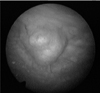
A 72-year-old man presented to our urology department with a history of gross painless hematuria and dysuria. Initial laboratory signs were within normal levels, except hematuria in routine urine analysis. Abdominal computed tomography revealed a sessile bladder mass (dimensions, 1.0 cm×1.5 cm) at the bladder dome, but no perivesical infiltration or regional lymphadenopathy was observed. Cystoscopy results also showed a smooth sessile mass (dimensions, 1.0 cm×1.5 cm) at the bladder dome (Fig. 1). The patient underwent transurethral resection for treatment of the bladder mass. Histological analysis of the tissue submitted showed several fragments of bladder mucosa and subepithelial tissue. Microscopic examination showed several benign prostate glands with 2 distinct cell layers (the basal cell and luminal epithelial cell layers) embedded in the subepithelial stroma (Fig. 2). Some of the glands were cystically dilated (Fig. 3), and some contained corpora amylacea. There was no direct continuation with the bladder mucosa, and although some of the bladder mucosa was denuded, no severe inflammation was evident in the surrounding tissue. Immunohistochemical analysis results showed that most prostatic glands were strongly positive for prostate-specific antigen (PSA). The postoperative course was uneventful. The postoperative 6-month follow-up indicated that the patient was well and had not experienced a recurrence.
Most ectopic prostatic tissue that arises in the bladder involves the trigone and ureteral orifices.2 This is because of the characteristics of the embryonic prostatic elements. Although the origins of ectopic prostate tissue are not entirely clear, the frequent involvement of the tissue of the bladder trigone supports the theory of migration.5,6
Different theories have been proposed to explain this phenomenon, including the migration and misplacement of normal tissue, the persistence of embryonic remnants, and metaplastic change caused by chronic inflammation.5,6 In relation to ectopic prostatic tissue, the most likely explanation seems to be that the embryonic prostatic tissue migrated and became isolated.7 Ectopic prostatic tissue is histologically and immunohistochemically indistinguishable from normal prostatic tissue and most probably indicates the persistence of embryonic structures.2 Our case supports the migration theory because there was no histological evidence of inflammation or metaplastic processes in the subepithelial lesion.
The key complaints associated with this disease are microscopic or gross hematuria. The main point of differential diagnosis is bladder cancer relating to transitional cell carcinoma. One notable report demonstrated that ectopic prostate tissue is a potential site of hyperplastic and malignant changes by describing a case of prostatic adenocarcinoma arising in the ectopic prostate tissue at the bladder dome.3 To date, 4 cases of ectopic prostate tissue at the bladder dome have been published. It is important to recognize that different tissues may coexist with ectopic prostatic tissue, including prostatic adenocarcinoma and urachal remnants.3
When ectopic prostatic tissue is encountered, PSA and prostate-specific acid phosphatase are usually the first 2 immunohistochemical stains to be used. CD10 is considered a marker for tissues of mesonephric origin and is reported to be positive in the epithelial and basal cells of normal and hyperplastic prostatic acini.8 When prostatic adenocarcinoma is suspected to arise in the ectopic prostate tissue, immunohistochemical staining for p63 or 34βE12 is used for the detection of basal cells. Overexpression of α-methylacyl-CoA-racemase can also be effective in the diagnosis of adenocarcinoma.2
The prognosis of ectopic prostatic tissue is excellent because it is benign and the lesion will not recur if the tissue is completely removed.2
In conclusion, we have described an extremely uncommon type of tumor at the bladder dome, which was confirmed to be ectopic prostatic tissue. Ectopic prostatic tissue is histologically and immunohistochemically indistinguishable from normal prostatic tissue, and most likely indicates the persistence of embryonic structures. However, other characteristics of the tissue must also be considered to confirm the diagnosis of ectopic prostatic tissue at the bladder dome.
Figures and Tables
References
1. Bellezza G, Sidoni A, Cavaliere A. Ectopic prostatic tissue in the bladder. Int J Urol. 2005; 12:1066–1068.

2. Halat S, Eble JN, Grignon DJ, Lacy S, Montironi R, MacLennan GT, et al. Ectopic prostatic tissue: histogenesis and histopathological characteristics. Histopathology. 2011; 58:750–758.

3. Gardner JM, Khurana H, Leach FS, Ayala AG, Zhai J, Ro JY. Adenocarcinoma in ectopic prostatic tissue at dome of bladder: a case report of a patient with urothelial carcinoma of the bladder and adenocarcinoma of the prostate. Arch Pathol Lab Med. 2010; 134:1271–1275.

4. Richter S, Saghi N, Nissenkorn I. Supratrigonal ectopic prostate: case report and review of the literature. Urol Int. 1991; 46:96–98.

5. Wallace C, Creager AJ, Cappellari JO, Bergman S. Ectopic prostatic tissue in the uterine cerrix. Am J Surg Pathol. 2001; 25:1215–1216.

6. Roy C, Guth S, Gasser B, Le Bras Y, Tuchmann C, Saussine C, et al. Benign hyperplasia in ectopic prostatic tissue: a rare cause of pelvic mass. Eur Radiol. 1997; 7:35–37.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download