Abstract
Purpose
The aim of this study was to investigate the anti-inflammatory effects of a new herbal formula (WSY-1075) in a nonbacterial prostatitis rat model.
Materials and Methods
Prostatitis was induced in male Wistar rats (n=32) by treatment with 17 beta-estradiol and dihydrotestosterone for 4 weeks. After the induction of prostatitis, the rats were randomly divided into one of four treatment groups: control (n=8), ciprofloxacin (n=8), WSY-1075 (100 mg/kg) (n=8), and WSY-1075 (400 mg/kg) (n=8). After 4 weeks of treatment, the prostatic proinflammatory cytokine (tumor necrosis factor-α, interleukin [IL]-6, and IL-8) levels and histological findings were noted.
Results
The ciprofloxacin and WSY-1075 treatment groups showed significantly decreased proinflammatory cytokine levels compared with the control group. Histologically, treatment with ciprofloxacin and WSY-1075 significantly suppressed the severity of prostatitis lesions compared with those in the control group. No differences in the proinflammatory cytokine levels or histologic findings were observed with the dose dependent treatment of WSY-1075.
Conclusions
The new herbal formula, WSY-1075, showed effective anti-inflammatory activities in the prostate and may be useful for the clinical treatment of nonbacterial prostatitis. Our findings suggest that WSY-1075 has a beneficial effect on the prevention and treatment of nonbacterial prostatitis.
Nonbacterial prostatitis is the most common urological diagnosis in men under 50 years of age and is the third most common urologic diagnosis in men over 50 years of age. It is marked by perineal pain radiating to the genital area, urinary symptoms, and ejaculatory disturbance, which have great impacts on the psychological and physiological status and quality of life of patients. An estimated 50% of all men experience prostatitis-like symptoms at some point during their lifetime.1 The etiology and pathogenesis of nonbacterial prostatitis is unclear, so it is a difficult condition to treat. There is growing evidence that inflammation plays a significant role in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Thus, elevated levels of proinflammatory cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor alpha (TNF-α), and IL-8 have been associated with diagnosis and symptom severity in patients with CP/CPPS.2,3 For this reason, anti-inflammatory medications have been used for the treatment of CP/CPPS.
Rat models of hormone-associated nonbacterial prostatitis can be useful for elucidating the mechanisms of the pathogenesis of nonbacterial prostatitis.4 Wistar rats spontaneously develop nonbacterial prostatitis with advancing age, which makes them a good animal model for laboratory investigation of nonbacterial prostatitis.5 Administration of exogenous estradiol (E2) increases the incidence and severity of prostatitis in adult Wistar rats.5,6 Naslund et al5 reported that spontaneously-developed prostatitis and E2-induced prostatitis in Wistar rats had the same histologic findings. Other studies have demonstrated that spontaneous nonbacterial prostatitis in rats was histologically very similar to CP in humans.7,8
Herbal formulas such as Cervus nippon, Panax ginseng C.A. Meyer, Cornus officinalis, Angelica gigas, Lycium chinense, and Cinnamomum zeylanicum are widely known nutrient tonics in the general population. Their extracts, individually or in combination, have been shown to have anti-inflammatory effects.9-13 The aim of our study was to investigate the anti-inflammatory effects of a new multi-herbal formula called WSY-1075, composed of six herbs (C. nippon, P. ginseng C.A. Meyer, C. officinalis, A. gigas, L. chinense, C. zeylanicum) in the treatment of nonbacterial prostatitis in a rat model.
The herbal formula used in our experiments was WSY-1075. The major ingredients of herbal formula WSY-1075 were obtained from six plants: 10% C. nippon, 10% P. ginseng C.A. Meyer, 25% C. officinalis, 25% A. gigas, 25% L. chinense, and 5% C. zeylanicum. A mixture of the dried seeds of the six plants was extracted with tap water (0.25 g/ml) for 3 hour by boiling. The extracts were filtered, concentrated in vacuo, and lyophilized. A venture company, Korea Bio Medical Science Institute Co. Ltd. (Iksan, Korea), which is developing oriental herbal medicines, developed this product as a health supplement.
The experimental animals (12-week-old male Wistar rats) were purchased from Samtaco Bio, Co. (Osan, Korea). The rats were housed in an animal room maintained at a constant temperature and humidity with a 12-hour light/dark cycle. The treatment protocols were approved by the Institutional Animal Care and Use Committee (CUMC-2012-0124-01) and handled according to NIH guidelines. We used the modified model of Naslund et al5 and Robinette14 with estrogen treatment of male Wistar rats to induce inflammation in the lateral prostatic lobe.
The male Wistar rats were treated with 17 beta-E2 and dihydrotestosterone (DHT) for 4 weeks to induce nonbacterial prostatitis. For the induction of nonbacterial prostatitis, E2 and DHT were dissolved in sesame oil and injected subcutaneously at a dose of 250 µg/kg/day. E2 was injected subcutaneously into the back of each rat for 4 weeks, and DHT was added for 2 weeks from day 15. After 4 weeks of induction of nonbacterial prostatitis, the rats were randomly divided into one of four treatment groups, control (n=8), ciprofloxacin (n=8), WSY-1075 (100 mg/kg) (n=8), and WSY-1075 (400 mg/kg) (n=8), and received treatment by orogastric tube once a day for 4 weeks. After 4 weeks of treatment, the prostatic proinflammatory cytokine (TNF-α, IL-6, and IL-8) levels and histological findings were noted.
The rats were sacrificed on day 60, and the prostate was resected. For histologic evaluation, we dissected both lateral lobes of each prostate. Thereafter, the samples were fixed in a neutral 10% formalin solution for 18 hours at room temperature, dehydrated in ethanol, cleared in xylene, and embedded in paraffin. For routine histological analysis with hematoxylin and eosin, the prostate lateral lobe sections of the paraffin-embedded tissues were cut into 6-µm thicknesses. Four-micron sections were taken from each lateral lobe of each prostate and stained with hematoxylin and eosin. Tissues were considered positive for prostatitis if one area of inflammatory cell infiltration was observed in a microscopic section. The severity of inflammation was assessed according to the aggressiveness of inflammation and by counting the number of inflamed acini. Grade 0 meant no contact between the inflammatory cells and epithelium; grade 1, some contact; grade 2, periglandular infiltrates adjacent to partially destroyed epithelium; and grade 3, the presence of more than 25% acini. The number of inflamed acini was counted for the whole lateral prostate area using the same sections.
Blood obtained before sacrifice was centrifuged for 10 min (3,000 rpm, 4℃), and the supernatant was immediately transferred to a tube. The cytokine concentration was measured every 5 minutes for 30 minutes, using a spectrophotometer at 450 nm with an immunoassay ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
Extensive infiltration of inflammatory cells in the lumina, mononuclear cells in the stroma of the gland, and epithelial degeneration were observed in the control group, suggesting the presence of nonbacterial prostatitis. In the ciprofloxacin-treatment group, there were fewer inflammatory cells in the lumina, and the epithelial cells of the gland and stroma showed more improvement compared to the control group, at a level associated with grade 1 in all of the rats. In the WSY-1075 (100 mg/kg) and WSY-1075 (400 mg/kg) treated groups, the inflammatory cells in the lumina and the epithelial cells of the gland and stroma showed more improvement than the control group but less improvement than the ciprofloxacin group (Fig. 1).
To investigate the effects of WSY-1075 on nonbacterial prostatitis, analyses of the inflammatory cytokines IL-6, IL-8, and TNF-α were conducted. Rats in the ciprofloxacin, WSY-1075 (100 mg/kg), and WSY-1075 (400 mg/kg) treatment groups showed significant decreases in pro-inflammatory cytokine IL-6 when compared to the control group (239.1 pg/ml, 929.0 pg/ml, and 820.1 pg/ml vs. 1490.4 pg/ml) (p<0.05). The pro-inflammatory cytokine level of IL-8 of the ciprofloxacin, WSY-1075 (100 mg/kg), and WSY-1075 (400 mg/kg) treatment groups showed significant decreases when compared to the control group (94.6 pg/ml, 285.4 pg/ml, and 247.9 pg/ml vs. 68.8 pg/ml) (p<0.05). The pro-inflammatory cytokine level of TNF-α of the ciprofloxacin, WSY-1075 (100 mg/kg), and WSY-1075 (400 mg/kg) treatment groups showed significant decreases when compared to the control group (25.3 pg/ml, 44.7 pg/ml, and 48.8 pg/ml vs. 68.8 pg/ml) (p<0.05). The ciprofloxacin treatment group showed significant decreases in pro-inflammatory cytokines when compared to the WSY-1075 treatment groups (p<0.05). (Fig. 2, 3, 4). There were no significant differences between the pro-inflammatory cytokine decreases in the WSY-1075 (100 mg/kg) and the WSY-1075 (400 mg/kg) groups.
We determined that treatment with WSY-1075 decreased the expression of pro-inflammatory cytokines in a nonbacterial prostatitis rat model. We showed that WSY-1075 reduced TNF-α, IL-6, and IL-8 concentrations in the prostate tissue of nonbacterial prostatitis rats. TNF-α secreted from macrophages is a trigger for the migration of activated macrophages and the production of several chemokines including IL-8 in inflammatory foci. Elevated IL-8 levels in the stroma of the prostate result in the accumulation of neutrophils and lymph cells, which suppress H2O2 production.15 IL-8 also seems to be a key mediator in human benign prostatic hyperplasia (BPH). IL-8 concentrations in prostatic secretions from patients with BPH with inflammation are higher than in patients with BPH alone.16 Penna et al3 reported that IL-6 and IL-8 are significantly elevated in the semen and expressed in the prostatic secretions of men with CP/CPPS, and their levels are positively correlated with symptom scores. Taken together, these findings support the association of nonbacterial prostatitis with increased levels of proinflammatory cytokines.
Treatment with the multi-herbal medicine WSY-1075 led to decreased inflammation scores for the prostates from our nonbacterial prostatitis rat model. These effects are presumed to be the result of the anti-inflammatory effects of WSY-1075. Although an appropriate animal model that mimics human BPH/lower urinary tract symptoms (LUTS)/inflammation has not yet been established, some hormone-induced models produced by combined treatment with E2 and testosterone or by treatment with E2 alone in castrated rats have been proposed to be useful for elucidating the mechanisms of the molecular pathology of nonbacterial prostatitis.4 Combined treatment with E2 and testosterone results in a significant increase in prostate weight and induces distinct prostatic stromal changes, although although some reports show that testosterone, with its anti-inflammatory effects, attenuates E2-induced increases in the incidence and severity of prostatitis.5
We applied the 4-point inflammation grading system used by Bernoulli et al17 to evaluate the severity of inflammation of the lateral prostate lobes. The severity of inflammation was assessed according to the aggressiveness of inflammation and by counting the number of inflamed acini from grade 0 to grade 3. In microscopic findings, reduced inflammation of the prostate and relieved degeneration of of the glandular epithelium were shown in the WSY-1075-treated rat group compared to those in the control group.
There was no dose-dependent relationship between WSY-1075 and pro-inflammatory cytokine levels or histologic findings.
The etiology and pathogenesis of nonbacterial prostatitis remains unclear. Causative factors that have been reported are Trichomonas vaginalis, Chlamydia trachomatis, genital mycoplasmas, staphylococci, coryneforms, genital viruses, biofilms, stagnation of prostatic secretion, autoimmune diseases, allergies, sex hormone disorders, and psychological factors.18-20 Even though several treatment strategies have been applied, it is still difficult to treat nonbacterial prostatitis. A recent report suggests that chronic nonbacterial inflammation in the prostate plays a pivotal role in the symptom progression of BPH.21 Analysis of prostate biopsies in a subgroup of patients randomly selected from the Medical Therapy of Prostate Symptoms (MTOPS) study indicates that the presence of inflammatory infiltrates in the prostates of patients with BPH is associated with increased progression and a higher risk of acute urinary retention.22 The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) study demonstrated a significant correlation between BPH-associated prostate inflammation and LUTS.23
The herbal formula WSY-1075 used in our experiments was prepared from a combination of extracts from C. nippon, P. ginseng C.A. Meyer, C. officinalis, A. gigas, L. chinense, and C. zeylanicum. In Korea, C. nippon extract is widely used for the long-term management and treatment of rheumatoid arthritis. Kim and Kim24 reported that C. nippon extract inhibited production of IL-1beta and TNF-alpha from macrophages in response to in vivo stimulation with bacterial lipopolysaccharides when the extract was administered to mice once a day for 7 days. P. ginseng C.A. Mayer (Araliaceae), one of the most popular herbal medicines, has been widely used for many traditional therapies in Korea, Japan, and China. Lee et al11 reported that the head butanolic fraction of ginseng showed anti-inflammatory effects. Jung et al25 demonstrated the anti-inflammatory effects of ginsenoside Rh1 in lipopolysaccharide (LPS)-stimulated microglia using in vitro and in vivo model systems. C. officinalis is a Korean traditional medicinal herb with tonic, analgesic, and diuretic activity and has been commonly used to facilitate liver and kidney function, reduce urination, and decrease perspiration. Sung et al26 reported that aqueous extracts of C. officinalis have anti-inflammatory and analgesic activities in murine RAW 264.7 macrophage cells. A. gigas, known by the Korean name cham-dang-gui, has been traditionally used in Oriental medicine. The pharmacological effects of A. gigas include anti-tumor activity, antiplatelet aggregation, Helicobacter pylori-suppression, neuroprotection, and memory enhancement.27,28 Earlier studies have demonstrated that another species of Angelica, Angelica acutiloba Kitakawa (Japanese angelica), has analgesic and anti-inflammatory effects.29 Kim et al30 reported that A. gigas was shown to inhibit induction of inflammatory mediators from LPS-stimulated macrophages. L. chinense is a traditional herbal drug that has been used since ancient times in eastern Asia for protective action of liver functioning and as a cure for diabetes mellitus. Currently, it is prescribed as an herbal medicine for the treatment of chronic hepatitis and liver cirrhosis. Previous studies have shown that L. chinense has cytoprotective effects against oxidative stress-induced hepatotoxicity and immunomodulatory effects.31,32 Oh et al12 reported that L. chinense has anti-inflammatory activity in LPS-stimulated RAW 264.7 mouse macrophage cells.
Experimental evidence clarifying the mechanism of WSY-1075 is limited at best. Many studies have reported on the anti-inflammatory effects of herbal formulas similar to the one that we used, but none evaluated the anti-inflammatory effects of these herbal medicines in nonbacterial prostatitis. To the best of our knowledge, this study is the first attempt to investigate the effects of herbal formula WSY-1075 on nonbacterial prostatitis in a rat model. We demonstrated that herbal formula WSY-1075 improves E2-induced nonbacterial prostatitis in rats by regulating pro-inflammatory cytokines but had less anti-inflammatory effect than antibiotic treatment. Since E2-induced nonbacterial prostatitis in rats is histolopathologically very similar to nonbacterial prostatitis in humans, WSY-1075 is expected to have beneficial effects on the prevention and treatment of nonbacterial prostatitis in human patients.
Results from our current study suggest that the new herbal formula, WSY-1075, which was prepared from a mixture of C. nippon, P. ginseng C.A. Meyer, C. officinalis, A. gigas, L. chinense, and C. zeylanicum extracts, may have anti-inflammatory effects in a nonbacterial prostatitis rat model. Our finding that WSY-1075 treatment significantly suppressed pro-inflammatory cytokines (TNF-α, IL-6, IL-8) in the rat model of nonbacterial prostatitis suggests that this new herbal formula may be useful for the clinical treatment of CP/CPPS in humans as well as contributing to the amelioration of prostate inflammation in BPH.
Figures and Tables
Fig. 1
Histopathologic findings of the prostate lateral lobe in each of the 4 groups (H&E, ×100). (A) The control group showed extensive infiltration of inflammatory cells, including neutrophils, lymphocytes, macrophages, and degeneration of glandular epithelial cells. All of the rats showed grade 3 inflammation. (B) The ciprofloxacin group showed a nearly normal appearance of the glandular epithelium with less leukocyte infiltration. All of the rats showed grade 1 inflammation. In the (C) WSY-1075 (100 mg/kg) and (D) WSY-1075 (400 mg/kg) groups, the inflammatory cells in the lumina and the epithelial cells of the gland and stroma showed more improvement than those of the control group but less improvement than those of the ciprofloxacin group. All of the rats showed grade 1~2 inflammation.
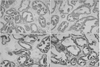
Fig. 2
Effect of WSY-1075 (100 mg/kg) and WSY-1075 (400 mg/kg) on serum tumor necrosis factor-α levels. Each value represents mean±standard deviation. *p<0.05, compared with the control group, †p<0.05, compared with the ciprofloxacin group.

ACKNOWLEDGEMENTS
This study was supported by a grant from the Traditional Korean Medicine Project, Ministry of Health & Welfare, Republic of Korea (commercialize TKM products, F110004).
References
1. Fowler JE Jr. Prostatitis. In : Gillenwater JY, Grayhack JT, Howard SS, Duckett JW, editors. Adult and pediatric urology. 2nd ed. St. Louis: Mosby Year Book;1991. p. 1395–1423.
2. Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, et al. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000; 164:214–218.
3. Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007; 51:524–533.

4. Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007; 10:15–29.

5. Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988; 140:1049–1053.

6. Seethalakshmi L, Bala RS, Malhotra RK, Austin-Ritchie T, Miller-Graziano C, Menon M, et al. 17 beta-estradiol induced prostatitis in the rat is an autoimmune disease. J Urol. 1996; 156:1838–1842.

7. Lundgren R, Holmquist B, Hesselvik M, Müntzing J. Treatment of prostatitis in the rat. Prostate. 1984; 5:277–284.

9. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, et al. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009; 58:431–436.
10. Park CH, Noh JS, Kim JH, Tanaka T, Zhao Q, Matsumoto K, et al. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol Pharm Bull. 2011; 34:1559–1565.

11. Lee JH, Lee JH, Lee YM, Kim PN, Jeong CS. Potential analgesic and anti-inflammatory activities of Panax ginseng head butanolic fraction in animals. Food Chem Toxicol. 2008; 46:3749–3752.
12. Oh YC, Cho WK, Im GY, Jeong YH, Hwang YH, Liang C, et al. Anti-inflammatory effect of Lycium Fruit water extract in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Int Immunopharmacol. 2012; 13:181–189.

13. Kim KW, Kim KS, Park SD, Kim JK, Chung KH, Kim DS, et al. Effect of Cervus korean TEMMINCK var. mantchuricus Swinhoe on protease activities, antioxidant and free radical damages in rheumatis arthritis rats. Toxicol In Vitro. 2008; 22:80–86.

14. Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988; 12:271–286.

15. Oka M, Tachibana M, Noda K, Inoue N, Tanaka M, Kuwabara K. Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of eviprostat, a phytotherapeutic agent for benign prostatic hyperplasia. Phytomedicine. 2007; 14:465–472.

16. Liu L, Li Q, Han P, Li X, Zeng H, Zhu Y, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009; 74:340–344.

17. Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate. 2008; 68:1296–1306.

19. Arakawa S, Matsui T, Gohji K, Okada H, Kamidono S. Prostatitis--the Japanese viewpoint. Int J Antimicrob Agents. 1999; 11:201–203.

20. Donovan DA, Nicholas PK. Prostatitis: diagnosis and treatment in primary care. Nurse Pract. 1997; 22:144–146.
21. Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007; 100:327–331.

22. Roehrborn CG. Definition of at-risk patients: baseline variables. BJU Int. 2006; 97:Suppl 2. 7–11.

23. Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999; 84:976–981.

24. Kim J, Kim K. Inhibitory effects of cervi pantotrichum cornu herbal acupuncture on type II collagen-induced arthritis. J Korean Acupunct Moxibustion Soc. 2002; 19:155–170.
25. Jung JS, Shin JA, Park EM, Lee JE, Kang YS, Min SW, et al. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem. 2010; 115:1668–1680.

26. Sung YH, Chang HK, Kim SE, Kim YM, Seo JH, Shin MC, et al. Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells. J Med Food. 2009; 12:788–789.

27. Ahn KS, Sim WS, Lee IK, Seu YB, Kim IH. Decursinol angelate: a cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Med. 1997; 63:360–361.
28. Bae EA, Han MJ, Kim NJ, Kim DH. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull. 1998; 21:990–992.

29. Cho S, Takahashi M, Toita S, Cyong J. Suppression of adjuvant arthritis on rat by oriental herbs. Shoyakugaku zasshi. 1982; 36:78–81.
30. Kim JH, Jeong JH, Jeon ST, Kim H, Ock J, Suk K, et al. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-kappaB activation in macrophages. Mol Pharmacol. 2006; 69:1783–1790.
31. Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD, Chae S, et al. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J Ethnopharmacol. 2010; 130:299–306.
32. Amagase H, Sun B, Nance DM. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food. 2009; 12:1159–1165.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download