Abstract
Purpose
The purpose of the present study was to investigate the effect of sesame oil on the reproductive parameters of diabetic male Wistar rats.
Materials and Methods
The adult male rats in a split plot design were divided into normal (n=10), normal 5% (n=5; 5% sesame oil enriched diet), diabetic (Streptozocin induced diabetes; n=9), diabetic 5% (n=9; 5% sesame oil enriched diet), and diabetic 10% (n=9; 10% sesame oil enriched diet) groups. Diet supplementation continued for 56 days.
Results
Sesame oil supplementation did not reduce the plasma glucose concentration of rats in the diabetic groups (p>0.05). The total spermatogonia, spermatocytes, Leydig cells/tubule, and the germ cell to Sertoli cell ratio were lower in the diabetic rats than the normal ones (p<0.05), and with the exception of spermatogonia counts, these values improved by the addition of sesame oil to the diet (p<0.05). The sperm progressive motility and viability were lower in the diabetic rats (p<0.05) and sesame oil supplementation did not improve them. Incorporation of sesame oil into the diet improved the plasma testosterone concentration of the diabetic rats in a dose-dependent manner (p<0.05).
The effects of diabetes on male reproduction have been documented.1-6 Impairment of sexual behavior,5 semen quality,2 and ejaculation7 are the most common reproductive consequences in diabetic men. Disrupted spermatogenesis and oligozoospermic cases of diabetes have also been reported.8,9 Decreased sperm motility1 and disturbance in gonadal10 and gonadotropin1 hormones were reported in diabetics as well. Two different mechanisms have been suggested for the reproductive complications of diabetes: endocrine neuropathies11 and metabolic disturbances leading to oxidative stress.12 Diabetes mellitus is usually accompanied by extensive disturbances in the metabolism of glucose and fatty acids.13 The extensive impacts of lipid peroxidation in the testis and epididymal sperm of streptozocin (STZ)-induced diabetic rats have been demonstrated previously.14
Different drugs and compounds may reduce the reproductive consequences of diabetes. Ginseng extract supplementation,15 nano-structures,16 Zingiber officinale root,17 rosiglitazone,18 metformin,19 and insulin supplementation20 improved the reproductive performance and sperm quality of diabetics. Sesamum radiatum leaves improved the testicular structures of normo-glycemic adult males.21 It has been suggested that the effects of sesame leaves can be mediated through the antioxidative properties of their lignans.22-24 Sesame oil is beneficial in improving the blood glucose, glycosylated hemoglobin, lipid-peroxidation, and antioxidant levels in female STZ-induced diabetic rats.21,22,25
The aim of the present study was to investigate whether sesame oil supplementation can reduce the impact of diabetes on testicular structures and function, specifically the testicular micro-structures, sperm parameters, and hormone profile, of STZ-induced diabetic rats.
Male Wistar rats were purchased from the animal facility of Shahid Chamran University of Ahvaz. STZ was acquired from Pharmacia and Upjohn (Germany). All chemicals were from Merck (Germany). The sources of other materials have been specified throughout the text.
The animals were harbored in stainless steel cages under standard laboratory conditions of a 12 hours light/dark cycle throughout the experimental period with ad libitum access to food and water. Supplementation of the diet with sesame oil (v/w) in the respective groups was performed by mixing an adequate volume of oil and powdered food. The rats were housed at a controlled temperature of 23±2℃ schedule and their health was carefully monitored every day. For the animal care, the ethics guidelines for using laboratory animals in experiments published by Tehran University of Medical Sciences was followed throughout the study (http://mehr.tums.ac.ir/ShowCode.aspx?CodeID=104&lang=en).
Diabetes was induced by intravenous administration of citrate buffered (0.1 M, pH=4.5) STZ (50 mg/kg; body weight). One week after induction, the concentration of blood glucose was measured by a glucometer (Glucose assay tape; Bayonim, Berneck, the Netherlands), and the rats with a glucose concentration greater than 300 mg/dl were classified as diabetic. A similar volume of only citrate buffer (0.5 ml/kg; body weight) was intravenously infused in the rats assigned to the non-diabetic groups.
At the end of the experiment, the animals were euthanized and blood samples were collected in EDTA-coated glass tubes, centrifuged at 3,500 rpm for 15 minutes, and the separated plasma was stored at -20℃ until the glucose and hormone assays. The samples were assayed for glucose by the glucose oxidase method using a commercially available kit (glucose assay kit, Pars Azmun, Iran). The plasma testosterone and estradiol concentrations were measured using enzyme-linked immunosorbent assay (ELISA; DRG Instruments GmbH, Marburg, Germany). The intra- and inter-assay coefficients of variation for testosterone were 4.16 and 9.94, and for estradiol were 6.81 and 7.25, respectively.
The scrotum was cut with fine scissors and the testis-epididymis was removed. The testes and epididymides were dissected and weighed using a digital electronic balance. The testis and epididymis length and width were measured using a plastic tape. The testis was fixed in buffered-formalin (10%) and embedded within paraffin. The paraffin sections (5-µm thickness) were prepared and stained with H&E. The specimens were examined under light microscopy (Olympus/3H, Tokyo, Japan).
The left caudal epididymis was separated and the total recovered sperm during 4 h of incubation in normal saline (volume=1 ml, 35~37℃) was calculated. The sperm concentration was determined by the conventional method using a hemocytometer chamber for the red blood cell count. The right epididymis was finely minced by anatomical scissors in 1 ml of warmed isotonic saline in a petri dish. The sperm progressive motility (SPM) was estimated by evaluating 4 fields of a sperm droplet under a cover-slip on a warm glass slide (35~37℃) under light microscopy (×40). The sperm vitality was assayed using a conventional procedure of eosin B-nigrosin stain (1.67% eosin, 10% nigrosin, and 0.1 M sodium citrate) under ×100 magnification and 100 sperm were counted. Later, the morphological abnormalities of sperm were considered and recorded. All of the sperm evaluation procedures were carried out based on the World Health Organization manual for human sperm analysis26 with some modifications.
Five slides were selected from each group and 5 seminiferous tubules within each slide were evaluated for spermatogonia (spermatogonia A, intermediate, and B), spermatocytes (primary and secondary spermatocytes) and the Sertoli cell counts, and the Leydig cell numbers were evaluated in the interstitial tissue.27 The average of the different cell counts of each slide was used for the analysis. The evaluation of all of the samples was performed at a constant magnification of 40× with light microscopy. The ratio of the germ cell to the Sertoli cell number was calculated by dividing the sum of the counted spermatogonia and spermatocytes by the Sertoli cell numbers per tubule.
Forty-two diabetic and non-diabetic rats (190±10 g) were assigned into five groups; normal (n=10): the non-diabetic rats without any treatment, diabetic (n=9): the diabetic rats with no treatment, diabetic 5% (n=9): the diabetic rats that received a diet with 5% sesame oil (Saman, Tehran, Iran), diabetic 10% (n=9): the diabetic rats that received a diet with 10% sesame oil, and normal 5% (n=5): the non-diabetic rats that received a diet with 5% sesame oil. The experimental period lasted for 56 days to cover the period of a complete pathway of spermatogenesis in the rat.28
The univariate normal plot procedure was used for normality of data. Except SPM, the other parameters had a normal distribution. The arcsine transformation was used for normalizing the data on the SPM. The main effects of diabetes (diabetics and normal) and diet supplementation (with sesame oil or not) on the general parameters, serum glucose, T2 and cortisol concentrations, and testicular cells were analyzed by one way analysis of variance using a Generalized Linear Model (GLM) procedure in SAS software (Statistical Analysis System 9.1.3; SAS Institute, Cary, NC, USA). The Duncann's multiple range test was used to make all possible pairwise comparisons. The results were expressed as least square mean (L.S. Mean) and the standard error of the means.
Table 1 shows the effects of sesame oil supplementation and diabetes on the general parameters, sperm parameters, and hormone and glucose assays in the experimental groups.
Diabetes (220.3±9.4) reduced the body weight (g) compared to the normal group (300.8±11.0; p<0.05). Sesame oil supplementation did not improve the body weight gain (Table 1). Testis weight (g), volume (mm3), and length and width (mm) did not differ between the groups (p>0.05). Diabetes (0.37±0.03) reduced the weight (g) of the epididymis compared to the normal (0.48±0.02) animals (p<0.05), and diet supplementation of diabetic rats with sesame oil (0.395±0.03 and 0.41±0.03; for 5% and 10% oil, respectively) improved the weight of the epididymis compared with normal rats (Table 1; p>0.05). The testis to body weight ratio in the diabetic animals was higher than in the normal ones (p<0.05) and oil supplementation did not affect it (p<0.05). The ratio of epididymis to testis weight was higher in the diabetic rats than the normal group (p<0.05), and oil supplementation did not improve it in the diabetic animals (p>0.05).
The total sperm count was not significantly affected in the diabetic and sesame oil supplemented diabetic rats compared to the normal animals (p>0.05). However, supplementation of the normal rats' diet with 5% sesame oil significantly increased the total sperm count compared to the other groups (Table 1; p<0.05). Diabetes significantly decreased the SPM (p<0.05). Supplementation of the diet with 5% sesame oil improved the SPM in both the diabetic (p<0.05) and normal animals (p<0.05), while it was not statistically different in the diabetic 10% compared to the non-treated diabetic group (p>0.05). Diabetes reduced the sperm vitality (p<0.05) and 5% oil supplementation increased the percentage of live sperm compared with the non-treated diabetic rats (p<0.05). The high oil supplementation diet reduced the live sperm percentage to a similar level as the non-supplemented diabetic rats (p>0.05). The most obvious sperm abnormality was detached heads, which was significantly higher (p<0.05) in the diabetic rats, and oil supplementation did not influence it (p<0.05).
Fig. 1 shows the micrographs of the testis sections of different groups. The mean spermatogonia number (per tubule) was significantly decreased by either diabetes or sesame oil supplementation (Table 2; p<0.05). The oil supplementation had no beneficial effect on the spermatogonia number in diabetics (p>0.05). The value of spermatocyte numbers per tubule was significantly decreased in the diabetics (p<0.05) and sesame oil increased the cell counts to the level of the normal rats (p>0.05). Inclusion of 5% sesame oil within the diet did not influence the number of spermatocytes per tubule compared to the non-treated normal animals (p>0.05). The mean Sertoli cell count was not influenced by either diabetes or oil supplementation (p>0.05). The mean Leydig cell count was significantly reduced in the diabetic rats and oil supplementation increased it in both the diabetic and non-diabetic rat testes (p<0.05). The germ cell to Sertoli cell ratio was lower in the diabetic animals compared to the other groups (p=0.03). Inclusion of 5% and 10% of sesame oil improved the ratio in the diabetic rats compared with the normal animals (p<0.05).
The plasma estradiol concentration was not influenced by diabetes (p>0.05). However, a tendency toward a non-significant decrease was observed in the plasma concentrations of the oil supplemented animals in both diabetic and non-diabetic rats (p=0.07). The mean plasma testosterone concentration was not affected in the diabetic rats compared to the normal animals (p>0.05). A high level of oil supplementation (10%) significantly increased the plasma testosterone concentrations in diabetic rats (Table 1; p<0.05). Enrichment of the diet with sesame oil, at any value, did not reduce the glucose concentration in diabetic rats (Table 1; p<0.05).
The dietary benefits of sesame oil and seeds have recently been reviewed by Namiki,23 who addressed the strong antioxidant properties of sesame oil and lignans such as sesamin and sesaminol, which are beneficial to humans. Moreover, the benefit of sesame seeds for menopausal women confirms the possible phytoestrogenic properties of its lignans29 through their interaction with sex steroids. The results of the present study have clearly shown that the impact of diabetes on several reproductive parameters of male rats was mitigated by sesame oil supplementation in term of increased plasma testosterone concentration and the germ cell to Sertoli cell ratio. However, oil supplementation did not improve the glucose concentrations or epididymal sperm parameters of diabetic rats. It did improve the sperm parameters in normal animals. An apparent tendency toward a decrease in plasma estradiol was also detected in the oil-supplemented animals.
The present study has demonstrated body weight loss in diabetic animals that is in line with other studies, which reported weight loss in diabetic cases.8,30 There are many studies which have reported the beneficial effects of some plant extracts31-33 on the body weight of diabetics. However, sesame oil supplementation did not alleviate the weight gain of diabetic rats. The impact of diabetes on the metabolic profile of STZ-induced diabetes has shown a decrease in the fatty acid metabolism mediators within the liver, such as L-carnitine.34 The effect of L-carnitine on the importation of fatty acids from the cytosol to the mitochondria for the oxidation process has been documented previously.35 This suggests that enrichment of the diet with an unsaturated fatty acid supplement such as sesame oil may worsen the metabolic disorders of diabetics. On the other hand, in normal animals with normal metabolism, a sesame oil-enriched diet may contribute to providing more useful materials for animal growth, as has been observed observed in normal animals with a 5% diet enrichment.
Surprisingly, in the present study the macroscopic parameters of the testis were not affected by either diabetes or oil supplementation. Moreover, the increased ratio of the testis to body weight, in the present study, is mostly related to the decrease in body weight rather than the increase in testis weight. This finding is in contrast with the previous studies that reported a decline in the testicular weight of diabetics.30,36 The difference can be due to the procedure of diabetes induction and testis weight measurement; in the present study, the epididymis was dissected from the testis and then weighed in chronically treated animals. However, the deposition of fat instead of depleted germinal cells may contribute to the establishment of the testicular weight in diabetics. Enrichment of normal animals' diet with 5% of sesame oil also did not increase the weight of the testis. This finding also disagrees with that of a previous report in which sesamum leaf gavages increased the testis weight of normoglycemic rats.21 Once again, these results may be based on the fact that the influence of oil-based sesame supplementation, in the disrupted metabolic system of diabetics, can be different from the consumption of the sesame leaves, hydro- or alcoholic extracts, and oil extract.
The epididymis was significantly influenced by diabetes. Soudamani et al20 have shown the atrophic changes in the different parts of the epididymides mostly due to decreased tubular diameter, volume, and surface density in diabetic rats. The enrichment of the diet with sesame oil improved the epididymis weight in a dose-dependent fashion.
While the total retrieved sperm in the present study was not influenced in the diabetic animals, another study reported the impact of the diabetes on the epididymal sperm.4 The difference may be due to different procedures for epididymal sperm collection. In the present study, 56 days after inducing diabetes, the sperm were retrieved from one side of the epididymides. We found that inclusion of 5% sesame oil in the diet of the normal rats increased the number of sperm cells significantly.
The impact of diabetes on sperm motility, viability, and abnormalities, in the present study, is in agreement with previous studies.4,6,37 Low-level oil supplementation (5%) in diabetics improved sperm motility and viability compared to diabetics without supplementation, which were significantly lower than in normal animals. Incorporation of 10% sesame oil within the diet of diabetics has no beneficial effect on sperm motility and viability. The concentration-dependent effect of sesame oil on the sperm parameters may be based on the nature of sesame oil. Sesame oil, despite its potent antioxidative properties, is rich in polyunsaturated fatty acids. It can be assumed that in chronic metabolic diseases such as diabetes, the extensive disturbances in lipid and glucose metabolism may reduce the efficacy of oil-based antioxidative agents, which was manifested in the present study by the lack of a decline in plasma glucose concentrations.
A significant decrease in the total number of spermatogonia and spermatocytes was observed in the diabetic animals and enrichment of the diet with sesame not only did not improve the spermatogonia numbers in the diabetics, but also significantly reduced their counts in normal animals. However, sesame oil increased depleted numbers of spermatocytes in the seminiferous tubules of the diabetic animals. In the present study, the Sertoli cell number/tubule was not affected. Diabetes decreased the Leydig cell count, and incorporation of sesame oil significantly increased their count, especially with 10% supplementation. The germ cell to Sertoli cell ratio was significantly lower in the diabetics, but was improved by both levels of oil enrichment.
A non-significant decreasing trend in the plasma estradiol was observed in the present study, where oil was incorporated within the diet. Sesame oil, because of its phytoestrogenic components,23 may exert negative feedback on the hypothalamus-hypophysis-testis axis and contribute to a decreasing trend in endogenous estradiol levels. However, this finding coincided with a non-significant decreasing trend in the Sertoli cell numbers by including oil within the diet of normal and diabetic animals. The marked increase in the serum testosterone in the rats with oil-enriched diets in this study may correlate with the proliferation of the Leydig cell count. The depletion of Leydig cells may lead to lower testosterone concentrations in diabetics. Testosterone deficiency produces immature sperm by early sloughing of spermatids from the Sertoli cells.38 However, in the present study, the most notable abnormality of the epididymal sperm was detached heads rather than round spermatids (data not shown). Improving the ratio of germ cells to Sertoli cells, in the present study, coincided with an increased plasma concentration of testosterone and the percentage of sesame oil in the diet may confirm the regulatory effect of testosterone on the Sertoli germ cell adhesion mechanism.39
In general, the results of the present study showed that incorporation of sesame oil into the diet may improve the reproductive parameters at the level of the testicular microstructures (germ cell to Sertoli cell ratio) and endocrine function (plasma testosterone concentration) without any beneficial effect on the epididymal sperm parameters in STZ-induced diabetic rats. Sesame oil supplementation was effective in improving reproductive parameters in normal rats as well.
Figures and Tables
Fig. 1
Microscopic views of testicular sections of different groups stained with H&E (magnification: ×20): Normal (A), diabetic (B), normal with 5% sesame oil (C), diabetic receiving 5% sesame oil (D), and diabetic receiving 10% sesame oil (E).
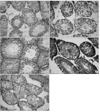
Table 1
The effects of sesame oil on general and sperm parameters and endocrine assays inmale diabetic Wistar rats*

Table 2
The effects of sesame oil on the cellular composition of the testis in the male diabetic Wistar rats*

*Values are presented as least square mean±standard mean. †Include all different types of spermatogonia (A, intermediate and B) within tubule. ‡Include all types of spermatocytes (primary and secondary) within tubule. $Calculated as (Spermatogonia+ Spermatocyte)/Sertoli cell. ∥, ¶, **,††Values with a common mark within rows are not significantly different (p>0.05).
ACKNOWLEDGEMENTS
The authors thank the research deputy of Shahid Chamran University of Ahvaz for funding the study.
References
1. Baccetti B, La Marca A, Piomboni P, Capitani S, Bruni E, Petraglia F, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002; 17:2673–2677.

2. García-Díez LC, Corrales Hernandez JJ, Hernandez-Diaz J, Pedraz MJ, Miralles JM. Semen characteristics and diabetes mellitus: significance of insulin in male infertility. Arch Androl. 1991; 26:119–128.
3. Mallidis C, Agbaje IM, Rogers DA, Glenn JV, Pringle R, Atkinson AB, et al. Advanced glycation end products accumulate in the reproductive tract of men with diabetes. Int J Androl. 2009; 32:295–305.

4. Navarro-Casado L, Juncos-Tobarra MA, Cháfer-Rudilla M, de Onzoño LÍ, Blázquez-Cabrera JA, Miralles-García JM. Effect of experimental diabetes and STZ on male fertility capacity. Study in rats. J Androl. 2010; 31:584–592.

5. Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 2006; 29:482–488.

6. Vignon F, Le Faou A, Montagnon D, Pradignac A, Cranz C, Winiszewsky P, et al. Comparative study of semen in diabetic and healthy men. Diabete Metab. 1991; 17:350–354.
7. Gilja I, Parazajder J, Radej M, Kovacic M, Reljanovic M. Diabetes and retrograde ejaculation: possibilities of conservative treatment. Diabetologia Croatica. 1996; 25:83–86.
8. Arikawe AP, Daramola AO, Odofin AO, Obika LF. Alloxan-induced and insulin-resistant diabetes mellitus affect semen parameters and impair spermatogenesis in male rats. Afr J Reprod Health. 2006; 10:106–113.
9. Noguchi S, Ohba Y, Oka T. Involvement of epidermal growth factor deficiency in pathogenesis of oligozoospermia in streptozotocin-induced diabetic mice. Endocrinology. 1990; 127:2136–2140.

10. Komaki K, Ohno Y, Aoki N. Gonadal hormones and gonadal function in type 2 diabetes model OLETF (Otsuka Long Evans Tokushima Fatty) rats. Endocr J. 2005; 52:345–351.

11. Ali ST, Shaikh RN, Ashfaqsiddiqi N, Siddiqi PQ. Serum and urinary levels of pituitary--gonadal hormones in insulin-dependent and non-insulin-dependent diabetic males with and without neuropathy. Arch Androl. 1993; 30:117–123.

12. Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res Clin Pract. 2011; 91:61–66.

13. Giroix MH, Louchami K, Carpentier YA, Sener A, Malaisse WJ. Fatty acid pattern of pancreatic islet lipids in Goto-Kakizaki rats. Endocrine. 2010; 37:173–179.

14. Shrilatha B. Muralidhara. Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int J Androl. 2007; 30:508–518.

15. Sawiress FA, Ziada MS, Bebawy WS, Amer HA. Effect of ginseng extract supplementation on testicular functions in diabetic rats. Endocr Regul. 2011; 45:139–148.

16. Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, et al. Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats. Toxicology. 2011; 282:69–81.

17. Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010; 48:2920–2924.

18. Rabbani SI, Devi K, Khanam S. Effect of rosiglitazone on the nicotinamide-streptozotocin induced type-2 diabetes mellitus mediated defects in sperm abnormalities and oxidative defense system in male Wistar rats. Acta Pharmaceutica Sciencia. 2010; 52:121–128.
19. Rabbani SI, Devi K, Khanam S. Effect of metformin against the nicotinamide-streptozotocin induced sperm abnormalities in diabetic male wistar rats. Biomed Pharmacol J. 2009; 2:31–38.
20. Soudamani S, Malini T, Balasubramanian K. Effects of streptozotocin-diabetes and insulin replacement on the epididymis of prepubertal rats: histological and histomorphometric studies. Endocr Res. 2005; 31:81–98.

21. Shittu Lukeman AJ, Shittu Remilekun K, Ogundife O, Tayo Adetokunbo O, Osunubi Abraham AA. Hypoglycaemia and improved testicular parameters in Sesamum radiatum treated normo-glycaemic adult male Sprague Dawley rats. Afr J Biotechnol. 2009; 8:2878–2886.
22. Arumugam P, Ramesh S. Protective effects of sesame oil on 4-NQO-induced oxidative DNA damage and lipid peroxidation in rats. Drug Chem Toxicol. 2011; 34:116–119.

23. Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007; 47:651–673.

24. Umeda-Sawada R, Ogawa M, Igarashi O. The metabolism and n-6/n-3 ratio of essential fatty acids in rats: effect of dietary arachidonic acid and a mixture of sesame lignans (sesamin and episesamin). Lipids. 1998; 33:567–572.

25. Ide T, Lim JS, Odbayar TO, Nakashima Y. Comparative study of sesame lignans (sesamin, episesamin and sesamolin) affecting gene expression profile and fatty acid oxidation in rat liver. J Nutr Sci Vitaminol (Tokyo). 2009; 55:31–43.

26. WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva, Swittzerland: WHO press;2010. p. 22–44.
27. McLachlan RI, Wreford NG, O'Donnell L, de Kretser DM, Robertson DM. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol. 1996; 148:1–9.

28. Van Beek ME, Meistrich ML. A method for quantifying synchrony in testes of rats treated with vitamin A deprivation and readministration. Biol Reprod. 1990; 42:424–431.
29. Wu WH, Kang YP, Wang NH, Jou HJ, Wang TA. Sesame ingestion affects sex hormones, antioxidant status, and blood lipids in postmenopausal women. J Nutr. 2006; 136:1270–1275.

30. Hassan AA, Hassouna MM, Taketo T, Gagnon C, Elhilali MM. The effect of diabetes on sexual behavior and reproductive tract function in male rats. J Urol. 1993; 149:148–154.

31. Irshaid F, Mansi K. Effects of leaf extract of Urtica pilulifera L. on male reproductive system of streptozotocin-diabetic rats. Am J Pharmacol Toxicol. 2009; 4:22–28.

32. Mallick C, Mandal S, Barik B, Bhattacharya A, Ghosh D. Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol Pharm Bull. 2007; 30:84–90.

33. Tahtamouni LH, Alqurna NM, Al-Hudhud MY, Al-Hajj HA. Dandelion (Taraxacum officinale) decreases male rat fertility in vivo. J Ethnopharmacol. 2011; 135:102–109.

34. Mallidis C, Agbaje I, O'Neill J, McClure N. The influence of type 1 diabetes mellitus on spermatogenic gene expression. Fertil Steril. 2009; 92:2085–2087.

35. Hao J, Shen W, Sun L, Long J, Sharman E, Shi X, et al. Mitochondrial dysfunction in the liver of type 2 diabetic Goto-Kakizaki rats: improvement by a combination of nutrients. Br J Nutr. 2011; 106:648–655.

36. Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrinemale reproductive tract axis of the adult rat. J Urol. 1987; 138:190–194.

37. Padrón RS, Dambay A, Suárez R, Más J. Semen analyses in adolescent diabetic patients. Acta Diabetol Lat. 1984; 21:115–121.

38. O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996; 55:895–901.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download