Abstract
Late-onset hypogonadism (LOH) is a syndromic condition that has a well-recognized association with sexual and reproductive failure. LOH is frequently associated with chronic conditions including cardiovascular diseases (CVD), obesity, osteoporosis, HIV infection, renal failure, and obstructive pulmonary diseases. Despite this evidence, in patients with these conditions, LOH is still only rarely investigated and testosterone replacement therapy (TRT) rarely considered. In this paper, we critically reviewed the available evidence on LOH treatment focusing on possible risks and benefits. Medical therapy of LOH should be individualized depending on the etiology of the disease and the patient's expectations. The fear of prostate cancer and the risk of erythrocytosis probably represent the main limitations of TRT in aging men. However, TRT in healthy older men in near physiological doses does not appear to incur serious adverse events, although regular monitoring of prostate-specific antigen and hematocrit levels is required. Available evidence also suggests that TRT might ameliorate central obesity and glycometabolic control in patients with metabolic syndrome and type 2 diabetes. In addition, TRT has been associated with an increase in bone mineral density in men with osteoporosis, with an improvement in lean body mass in subjects with human immunodeficiency virus infection or chronic obstructive pulmonary disease, as well as with peripheral oxygenation in patients with chronic kidney diseases. Despite this evidence, however, it should be recognized that the results of these trials were heterogeneous and limited by small sample sizes. Hence, further research is required regarding the long-term benefits and adverse effects of TRT in LOH.
The testis, the male gonad, has essentially a double function: 1) an excretory one, that is, the production of sperm cells (spermatogenesis) - which depends on the intact functioning of the seminiferous tubules - and 2) an endocrine function, that is, the secretion of sex steroids from the Leydig cells (steroidogenesis) - testosterone (T) being the main product.1 There is much evidence suggesting that the testicles produce other non-steroidal hormones, such as insulin-like 3 (INSL-3),1,2 but little is known about their physiological role. Spermatogenesis and steroidogenesis are not independent phenomena. Spermatogenesis is a highly complex process, involving subtle and continuous interactions between paracrine and autocrine regulators - among which T is the most important - and pituitary-derived hormones (gonadotropins: follicle-stimulating hormone [FSH] and luteinizing hormone [LH]). LH and FSH secretion from the anterior pituitary is finely regulated by inhibitory influences coming from the testis itself (sex steroids and inhibin B) and stimulated by the secretion in the portal vessels of the pituitary stalk of a decapeptide, gonadotropin-releasing hormone (GnRH), synthesized in the arcuate nucleus of the hypothalamus.1,2 The pulsatile secretion in the stalk vessels of GnRH is negatively controlled by the activity of other hypothalamic neurons, including corticotrophin-releasing hormone and β endorphin, and stimulated by Kiss-1 neurons, though the activation of the specific Kiss-1 receptor, G protein-coupled receptor-54.1,2 Infusion of Kiss-1 in humans stimulates GnRH secretion from the hypothalamus, leading to an increase in LH and FSH secretion and a corresponding rise in T.1,2
Production of T by the human fetal testis begins at approximately 8 weeks of gestation and peaks between 11 and 14 weeks of gestation.1,2 During the first year of life, plasma T concentrations decrease with time. Thereafter, testicular T secretion remains very low during childhood until puberty, when production of both adrenal and testicular androgens markedly increases, leading to androgen-dependent changes such as deepening of the voice, growth of pubic hair, and an increase in muscle bulk.1,2 In the adult male, sex steroids, and in particular T and its active metabolite 5α-dihydro-T (DHT), have numerous biological functions that are essentially devoted to stimulating or maintaining male characteristics and behaviors.1,2 The testes are the major source of circulating androgens and T is the principal androgen secreted. Besides T, the testes also secrete small amounts of other sex steroids including androstenedione, DHT, and estradiol. T plasma levels are relatively constant and sustained (high nanomolar range) because of a continuous testicular production. This relatively continuous T production is an essential condition for species perpetuation, allowing the male to be always ready to take advantage of sexual (and therefore reproductive) opportunities. In normal men, approximately 2% of circulating T is free or unbound, whereas the rest is either tightly bound to sex hormone binding globulin (SHBG) or loosely bound to albumin. Circulating SHBG (and thereby total T) is decreased by androgens and glucocorticoids or increased by estrogens and thyroxine.1,2 Other determinants of circulating SHBG levels are up-regulation by acute or chronic liver disease and androgen deficiency and down-regulation by obesity, insulin, and growth hormone deficiency.1,2 There is debate concerning which components of circulating T are capable of exerting a bioactive role. Free T (FT) is mostly considered the biologically active form of this hormone and, thus, the FT level is a better representation of the true T status.1,2 However, some clinicians believe that bioavailable T (T loosely bound to albumin+FT) is a better reflection of the true level of the active hormone than the level of FT alone.1,2 Although circulating T levels are relatively stable, small diurnal and seasonal fluctuations can be observed.1,2 For example, T plasma levels peak early in the morning and in summer. At least in younger men, early morning values are, on average, 30% higher than the trough levels later in the day. Seasonal fluctuation in T has also been documented and related to changes in air temperature via variation in hypophysial LH production.3 Although in modern societies air-conditioning, artificial lighting, and warm clothing attenuates abrupt seasonal changes, seasonal variation in the number of births has still been documented with a maximum during spring,4 which corresponds to a maximal conception rate during the time period when T reaches the highest levels. Sexual activity also affects T levels. Studies in the rhesus macaque indicate that plasma T levels are increased by exposure to receptive females and copulation.5 However, it is still unclear whether, in mating male individuals, T is higher to allow better sexual and reproductive fitness (affecting libido or penile erections and/or spermatogenesis) or the reverse is true: sexual activity positively affects T production.
In the present manuscript, we did not focus on the biological actions of T because the topic has already been covered by previous reviews.1,2 We should mention, however, that T exerts biological actions in part by itself, and in part through its reduction or aromatization to DHT and estrogens, respectively. Measurement of circulating DHT is often considered useless because it does not reflect target tissue production of the hormone, while measurement of estradiol (E2) in men is generally considered unreliable because the available assays are designed for detecting the ovulatory peak in women.6
Male hypogonadism is defined as the failure of the testes to produce sperm, sex steroids (T being the most abundant), or both, because of a distant (pituitary/hypothalamus) or a local (testicular) deficiency.1,6 The latter condition is termed primary (hypergonadotropic) hypogonadism, while the former is known as secondary or central (hypogonadotropic) hypogonadism. It is important to note that nowadays, in the andrology community, the term 'male hypogonadism' is commonly used to categorize T deficiency, which may or may not be associated with infertility. The classical dichotomic nosography in hypo- and hypergonadotropic hypogonadism retains a practical utility for treatment purposes. In fact, while hypogonadal patients with hypothalamic or pituitary diseases can be successfully treated with either gonadotropin/GnRH or T, for those affected by primary testicular failure, only T substitution may be considered. However, the aforementioned classification of hypogonadism (i.e., primary/secondary) does not take into consideration T-deficiency related symptoms. It is well known that both primary and secondary hypogonadism, if not treated, are characterized by symptoms and signs of dramatically different severity, ranging from a complete pseudo-female phenotype to no phenotype, dictated more by the age of onset of the testicular failure than by the cause of this failure. Hence, a new classification of male hypogonadism is needed. We now provide ours, based on time of symptom onset, as described in Fig. 1.7
In the case of very early-onset hypogonadism (VEOH), that is, during early fetal life, symptoms can be very severe, ranging from an almost completely feminine body shape (complete androgen insensitivity or enzymatic defects blocking androgen synthesis) to various defects in virilization (micropenis, hypospadias, cryptorchidism), such as in the case of impaired secretion or activity of GnRH or gonadotropins.6,7 In the case of a peri-pubertal appearance of hypogonadism (early-onset hypogonadism, EOH), because of central (e.g., pituitary tumors, such as germinoma) or peripheral defects (e.g., Klinefelter's syndrome), a slowing or delaying in the progression of puberty may occur, with a eunuchoidal phenotype, including scant body hair, a high-pitched voice, microorchidism, and prostate hypoplasia. EOH and, in particular, VEOH are often due to rather uncommon problems (although not so rare, spanning from 1:500 for Klinefelter's syndrome to 1:100,000 for complete androgen insensitivity syndrome). It is important to note that a clinical condition manifesting itself very early on in fetal life (VEOH) or before or during puberty (EOH) is dramatically different in terms of symptoms and signs from those beginning later on in adult life.6,7 The latter is called 'late-onset hypogonadism' (LOH), to denote a form of syndromic male hypogonadism with a clinical exordium in young adulthood or later on. LOH is the most frequent form of T deficiency.6,7 In the case of LOH, for any reason, hypogonadal symptoms will be relatively mild, insidious, and difficult to recognize, but often bothersome and frustrating, such as weakness and fatigue, reduced libido and erectile dysfunction, mood symptoms, low bone mineral density, and mild anemia, all of which can contribute to decreasing the overall quality of life. However, many of these symptoms are rather non-specific, as they are also characteristic of the aging process per se, which, in turn, may act as a confounder in the interpretation and identification of the syndrome. Fig. 1 also summarizes these concepts: VEOH and EOH are rare conditions strongly affecting phenotype and behaviors, while LOH is a rather common condition, however mild or crypto-symptomatic.
In contrast to what has been observed for female estrogen production - which essentially terminates abruptly after menopause - in the male, T levels slightly decline as a function of the aging process - in concert with many other biological functions - down to levels below the lower limit of younger individuals.6,7 In the Massachusetts Male Aging Study, after the age of 40, the average age-related reduction in total T is 0.8~1.6% per year, whereas FT declines with age by 1.7~2.8% per year.8 An unadjusted annual trend of decline for total T of 0.04 nmol/L per year (0.4% per year), has been observed in the European Male Aging Study (EMAS), a population-based study involving more than 3,000 subjects from eight European countries.9 In addition, in that study, healthy men had significantly higher median T concentrations at all time points of their life than apparently unhealthy men.9 Low T is, in fact, associated with several unhealthy conditions, such as impaired insulin sensitivity, increased body fat and visceral obesity, dyslipidemia, hypertension, and CVD. When this age- and/or morbidity-related T decline is associated with symptoms (fatigue, weakness, decreased libido and energy, erectile dysfunction) and signs (increased abdominal fat, reduced muscle and bone mass, decreased body hair, gynecomastia, small testis) it defines the clinical syndrome commonly referred to as LOH.6,10 In addition to LOH, the terms male climacteric, andropause, androgen deficiency in the aging male, partial androgen deficiency in the aging male, and T deficiency syndrome (TDS) have seldom been used to define this condition.6 Regardless of whether these terms were intended to do so, they have been interpreted as defining a unique hypogonadal syndrome in middle-aged or older men and have created controversy and confusion on the part of practicing clinicians and the lay public. Therefore, from a clinical standpoint, it is better to use the term LOH. LOH is a mixed form of hypogonadism, where a central (deficient GnRH and gonadotropin activity) and a peripheral (impaired steroidogenesis) component are often simultaneously present. Hence, in LOH gonadotropins are often apparently normal, but relatively inappropriate for low T.6
For diagnosing LOH, according to major international guidelines, two separate biochemical determinations of serum T levels must be obtained. In addition, as mentioned before, T levels should not only be low, but also associated with the presence of consistent symptoms and signs.6,10 Different T thresholds have been proposed for the biochemical definition of low T.6,10 The mostly shared consensus6,10 is that T substitution has to be offered to symptomatic individuals when circulating total T is below 8 nmol/L (231 ng/dl). In addition, there is also general agreement that a total T level above 12 nmol/L (346 ng/dl) does not require substitution. When total T is repetitively >8 and <12 nmol/L, in the presence of typical hypogonadal symptoms (as listed before), a T treatment trial might be considered.6,10 Although the measurement of FT represents the cornerstone of evaluating the androgen state, it is not an easy task, because the commercially available direct methods, based on a labeled T analogue, are often unreliable and separation of the unbound T fraction at equilibrium dialysis is much too technically difficult. Estimation of FT as calculated by Vermuelen's formula (at http://www.issam.ch/freetesto.htm) is therefore of great help. Guidelines proposed by the American Association of Clinical Endocrinologists11 and by the Endocrine Society12 differ in the T threshold suggested, considering a cut-off of 7 nmol/L (200 ng/dl) and 10.4 nmol/L (300 ng/dl), respectively. Recently, Wu et al13 introduced a new definition of LOH based on evidence-based data from the EMAS study. They found that many candidate symptoms, especially psychological symptoms, were not associated with decreased T levels in aging European men; however, they reported a non-linear threshold relationship between sexual symptoms and T levels. In particular, they demonstrated a syndromic association between decreased T levels (total T<11 nmol/L) and a triad of sexual symptoms: low libido and reduced spontaneous and sex-related erections.13 Hence, LOH should be diagnosed in the presence of at least three sexual symptoms and a morning total T level of less than 11 nmol/L (230~319 ng/dl) and FTT levels of less than 222.2 pmol/L (<64 pg/ml). By using the EMAS criteria, the prevalence of LOH in 40- to 80-year-old European men is much lower than previously reported, that is, 2.1%,13 with a rather similar figure when only the Florence subgroup was considered.14 Fig. 2 shows the prevalence of LOH in the authors' town (Florence, Italy) in subjects from the general population (n=433) and in those consulting our outpatient clinic for sexual dysfunction, which is, by definition, symptomatic of hypogonadism (n=3,293). The proportion of subjects identified as having EMAS-defined hypogonadism ranges from less than 5% in the youngest age group to 15% in the oldest age group. It is quite evident that in subjects complaining of sexual dysfunction, LOH is 5 times more prevalent than in the general population.
Each subject with LOH should be individually studied and counseled to carefully select the most valuable therapy for that problem in that particular time of a patient's life. No universal therapy for male hypogonadism exists, but many options should be carefully considered by the patient and his doctor. If the clinical and biochemical evaluation of low T leads to the diagnosis of a clear underlying condition that is amenable to treatment, this is the first option. In obese individuals, several studies have demonstrated that intense lifestyle intervention, along with nutritional counseling and physical activity, is able to reduce weight loss and to conjointly raise T levels. A recent meta-analysis, in fact, showed that weight loss is associated with an increase of bound and unbound T levels, along with gonadotropin levels, and that the final effect is directly related to the amount of body mass index (BMI) reduction.15 In addition, the rise in T levels was higher in younger and more obese subjects at baseline, whereas the prevalence of type 2 diabetes mellitus (T2DM) was inversely related with a weight-loss dependent increase in T levels.15 The nature of the hypogonadism (primary, secondary, or mixed) - along with patient needs - might also dictate the most appropriate treatment.2 For primary hypogonadism T substitution is the only choice. For secondary T deficiency, gonadotropins should be employed until fathering occurs. Antiestrogens are an alternative; however, their efficacy has not been adequately tested. In the presence of increased estrogen production symptoms (breast tenderness and gynecomastia), a short-term trial with non-aromatizable androgens (DHT, mesterolone or oxandrolone) might be advisable. However, after a few months of therapy, switching to other aromatizable preparations is recommended in order to prevent bone loss. T supplementation is the first choice in those without a short-term fertility objective. T replacement therapy (TRT) can be initiated on an individualized basis in LOH patients who have clinical signs and symptoms of androgen deficiency, if the benefits of treatment appear to outweigh the potential risks (see below) and only after a thorough discussion with the patient. Different preparations of T or other androgens are currently available in different countries, as reviewed elsewhere1,2 and summarized in Table 1.
The most universally known risk of TRT in older men is the development of prostate cancer (PC), one of the leading causes of death in males. Clinicians and regulatory agencies are all concerned by the fact that TRT could cause or promote PC and are very reluctant to prescribe or to approve TRT for the aging male. Huggins and Hodges, seven decades ago, originally developed and spread the clinical concept of the androgen dependence of PC, with the demonstration that castration caused the regression of PC.16 Later on, Wilson first hypothesized that the main androgen-inducing prostate overgrowth was not T, but its highly biologically active metabolite DHT, which is formed locally by the action of two 5α-reducing isoenzymes, 5α-reductase type 1 (5AR1) and 2 (5AR2), the latter being predominant.16 According to Wilson's original hypothesis, blocking DHT formation with type 2-selective (finasteride) or -unselective (dutasteride) inhibitors of 5α-reductase is an effective strategy in the medical treatment of benign prostatic hyperplasia. The fact that eunuchs and men with genetic deficiencies in 5α-reductase do not typically experience PC, along with the fact that androgen ablation causes PC regression, has long been cited to support a causal role of high androgen levels in PC development.16 Accordingly, the use of 5α-reductase inhibitors (5ARI) has been studied not only in BPH, but also as a chemopreventive strategy in PC. Both 5α-reductase isoenzymes are expressed in normal prostatic tissue, but in PC cells 5AR1 expression is increased and 5AR2 expression is decreased or unchanged, as compared to BPH tissue.16 Both 5ARI have been shown to decrease the risk of PC.16 Data from the PC Prevention Trial showed a 24.8% reduction in PC prevalence during the 7-year period between the finasteride (18.4%) and the placebo group (24.4%). The results of the Reduction by Dutasteride of PC Events (REDUCE) trial using dutasteride, has also been published in a large, randomized study to determine its ability to prevent PC. Over the 4 years of the trial, dutasteride, as compared to a placebo, reduced the relative risk of biopsy-detected PC by 23%. A total of 1,516 cancers were seen, with 659 in the dutasteride section and 857 in the placebo one. Over the course of the study, 6.8% of men in the placebo group and 6.7% of men in the dutasteride group were found to have aggressive, high-grade tumors, defined as a Gleason score of 7~10. The investigators found that there was no greater risk for the men who developed PC of having aggressive tumors. This outcome was closely watched because an earlier trial of a similar BPH drug - finasteride - produced controversial results with regard to the risk of more aggressive tumors in those men who developed PC while on finasteride.16
Although a causative role for circulating androgens in PC has been envisaged since the Huggins and Hodges studies, evidence-based data have clearly shown that such a link is at best unproven. We will now review evidence derived from epidemiological (a) and intervention (b) studies.
The most extensive analysis of all available epidemiological data found no evidence of a connection between androgens and PC. However, Parsons et al reported in the Baltimore Longitudinal Study of Aging that higher levels of calculated serum free T and a higher FT index were associated with an increased risk of PC, without a significant age-adjusted association for total T.16 In a prospective cohort study of 17,049 men (The Melbourne Collaborative Cohort Study), not only there was little evidence that androgen levels influenced the overall risk of PC, but the hazard ratio for aggressive cancer almost halved for a doubling of the concentration of T, suggesting that the androgen milieu is more protective than causal in PC.16 Finally, no associations were found between the risk of PC and serum concentrations of T, calculated FT, DHT, and other androgens in a meta-analysis of 18 prospective studies, including almost 4,000 men with incident PC and 6,500 control subjects released by the Endogenous Hormones and Prostate Cancer Collaborative Group.16 When interpreting these conflicting results, it should be taken into account that a major limitation of epidemiological studies in PC, a tumor which often afflicts aged men, is the lack of information on cumulative T exposure early on in mid-life, and in particular at a younger age. For instance, it is well known that African-American men have the highest incident rate of prostate cancer. Interestingly, the PC disparity between African- and Caucasian-Americans is evident as early as age 45, suggesting that T exposure in adulthood affects the trajectory of cancer risk later in life.16
Prostate development and size, and consequently prostate-specific antigen (PSA) levels, are known to be androgen-dependent. According to the Morgentaler and Traish hypothesis, the human prostate is sensitive to massive androgen ablation (castration levels), but rather insensitive in normal or even subnormal conditions (such as in mild hypogonadism) because, in physiological situations, the androgen receptor (AR) is 'saturated' by the circulating androgens and therefore quite unresponsive to further T increases.16 We recently confirmed this evidence in a large clinical series of subjects consulting for erectile dysfunction (ED). In particular, we found that PSA levels were androgen-dependent only in hypogonadal men, whereas this correlation disappeared with normal levels of T (Fig. 3).17,18 In particular, when the association between PSA and T was evaluated as a function of T deciles, the upper nine groups had similar PSA values, while the lowest demonstrated a significantly reduced PSA. Full prostate development during embryonic life requires a normal AR and an efficient synthesis of DHT; however, in later life, the correlation between androgen levels and prostate size is attenuated - because both prostate volume and PSA increase with aging - and verified only for the severe hypogonadal range of T concentrations. Therefore, a significant relationship is apparent only in the low T range and apparent in studies evaluating TRT in hypogonadal subjects, but not in those considering eugonadal subjects.16
In conclusion, at a physiological T concentration, no evidence suggests a link between androgen levels and PC risk, although there is no controversy over the fact that lowering the T serum to castration levels, at least temporarily, reduces PC progression.
A compilation of published prospective studies of TRT revealed only five cases of PC among 461 men (1.1%) followed up to 36 months, a prevalence rate similar to that in the general population. The pooled odds ratio for TRT derived from 19 randomized clinical trials was 1.09 (95% confidence interval [CI], 0.48~2.49) for PC and 1.19 (95% CI, 0.67~2.09) for PSA >4 ng/dl, or a 1.5% increase during the study.16 Another study, investigating the effect of TRT at the prostate level in aged hypogonadal subjects, clearly indicated that TRT has little effect on prostate cell function.16 In Marks's study, when TRT was compared to placebo, several androgen-sensitive genes, such as PSA, AR, NKX3.1, PAP2A, VEGF, and clusterin, measured by qRT-PCR, did not change in their prostatic expression, suggesting no effect of T substitution on prostate biology.16 However, it should be emphasized that these data, like all the data available in the literature, are derived from relatively short-term studies (maximum 36 months). It has been calculated that to detect a 30% difference in PC incidence between placebo and T-treated subjects, 6,000 older men with low T would need to be randomized to each treatment group and would require treatment for an average of 5 years.19
Animal and experimental models of PC have demonstrated a differential role of AR in inducing PC progression, depending on its location: differentiating in the epithelium and oncogenic in the stroma. Since PC arises from the epithelium, a beneficial more than a causative role for androgens is derived. Accordingly, the majority of in vitro studies in PC cells have shown that enforced expression of AR in otherwise AR-negative PC cells (as the PC-3 cell line) may decrease the metastatic/invasive potential of the cells.16
The risk of exacerbation of latent PC is always a key issue due to which a patient may be excluded from treatment if any suspicion of PC is present. Hence, according to all guidelines.6,12,13 TRT should not be prescribed to men with clinically evident PC, because the tumor is usually androgen sensitive. Guidelines suggest that men opting for T treatment be offered an estimation of PC risk based on PSA measurement and a digital rectal examination, at baseline. Men found to have a higher risk should have a urological examination before commencing T treatment even with PSA levels less than 4 ng/ml. While on treatment, the PSA levels should be monitored at 3 to 6 months after the initiation of treatment. An annual increment of PSA higher than 1.4 ng/ml should prompt a urological examination. Furthermore, an annual rate of PSA rise greater than 0.4 ng/ml over a 2-year period should also lead to a urological evaluation. However, based on the critical analysis of clinical trials and on the aforementioned experimental data on PC cell lines, several investigators have initiated TRT, even in PC patients, with the aim of inducing differentiation in the tumor biology.16 Table 2 summarizes those studies.20-29 In the vast majority of patients, an association with PC progression or clinical recurrence was not reported. Only one study, collecting records of 96 patients who received TRT after initial management for PC from 2000 to 2007, showed that nearly 60% of men had increasing PSA levels that triggered discontinuation of TRT, even though biochemical progression was not associated with clinical or symptomatic disease progression.25 In this series, however, the majority of PC subjects (61%) were treated, as a primary treatment, with androgen deprivation therapy and therefore a TRT-associated PSA rise was not surprising. In a recently published retrospective analysis,29 the authors reviewed the outcomes of 13 hypogonadal men (defined as the presence of typical symptoms and total T ≤10.4 nmol/L; median age, 68 years) with PC treated with brachytherapy or external beam radiotherapy and undergoing TRT. According to the National Comprehensive Cancer Network guidelines, the subjects were stratified into very low or low (n=4), intermediate (n=7), and high (n=1) risk of recurrence. TRT was based on a transdermal T formulation in 12 cases and T pellets in 1 patient. After a median follow-up of 29.7 months (range, 2.3~67.3), no significant change in PSA levels or evidence of PC recurrence was detected. However, it should be recognized that the number of reported cases is still small and heterogeneous. In the absence of randomized controlled trials (RCTs), the concept of using TRT for PC survivors is debatable. Accordingly, current recommendations suggest limiting TRT to symptomatic hypogonadal men successfully treated for PC, after a prudent interval, although the length of that interval is not specified.6,10
Prostate weight is only a few grams at birth, while it increases during puberty, reaching approximately 20 g in young adults. During puberty, there is extensive, androgen-dependent, prostate growth and remodeling, characterized by branching of ducts and development of new gland buds, followed by acini formation within the fibromuscular stroma. In contrast to the pubertal growth phase, which involves the entire gland, in about 75% of men, during the 5th decade of life, there is a second growth phase involving selectively one of the three anatomically distinct prostate zones, the periurethral one. Conversely, the other two prostate zones, that is, the peripheral and the central ones, which represent up to 95% of the total prostate volume, are generally unaffected. In humans, enlargement of the periurethral zone gives rise to the most common and costly age-related disease of the aging male; BPH. BPH is the most frequent tumor in aging men, with a histological prevalence at autopsy of 50% in men aged 50~60 years, and of 90% in those over 80 years. Although non life-threatening, BPH has been documented as substantially reducing quality of life, to an extent similar to hypertension and heart disease.30 The presence of androgens is required for the development of BPH and anti-androgen agents can decrease prostate volume in patients with BPH.30
Clinically, BPH is distinguished by progressive development of lower urinary tract symptoms (LUTS). BPH can cause LUTS because of compression of the prostatic urethra, which, in the earliest stages, is relatively compensated by the bladder muscular system. However, later on, the hypertrophic bladder becomes dysfunctional, complete voiding is prevented, and it finally decompensates with more frequent spontaneous activity and deterioration of the ability to generate pressure and empty. Chronic inflammation is another common finding in BPH.30 Epidemiological data have indicated that chronic inflammatory infiltrates are associated with higher prostatic volume, a higher risk of BPH progression, and acute urinary retention. We recently provided evidence that inflammatory infiltrates within the prostate are associated with metabolic syndrome (MetS) features, and, in particular, with dyslipidemia.31-35
Although it has been historically assumed that high T induces prostate overgrowth, most observational studies failed to find correlations between circulating T levels and BPH; in fact, no clear correlation with serum PSA or prostate volume across the normal T range has been shown.26 In addition, the notion that intraprostatic 5α-reductase activity, which is responsible for converting the bulk of T into DHT, is altered in the BPH tissues is contentious. However, it could be hypothesized that the intraprostatic level of DHT could be more important than the level of serum T for the growth of the prostate,16 thus explaining this lack of association between the serum T level and prostate overgrowth. Preclinical data from our lab demonstrate that, in an animal model of MetS,31,35-39 there was both prostate and bladder inflammation, which were further aggravated by hypogonadism and restored by T supplementation.35 In addition, we demonstrated that DHT exerts an immunoregulatory role in human prostatic BPH stromal cells, inhibiting their potential to actively induce and/or sustain autoimmune and inflammatory responses.40 This is relevant because the prostate is an immunocompetent organ, not only as it is populated by resident inflammatory cells, including T- and B-lymphocytes, macrophages, and mast cells, but also because stromal prostatic cells can secrete several pro-inflammatory cytokines and are able to recruit and activate CD4+ cells into the inflamed tissue.30 Under most conditions, this immunocompetence of the prostate would be beneficial to the host. However, in some situations, an immune response towards a Th1/Th17 cytokine profile might lead to the development of chronic immune-mediated tissue destruction and fibromyomatous growth, as observed in the pathogenesis of BPH. Interestingly, our data indicate that AR signaling might restrain, rather than facilitate, prostate inflammation. Thus, DHT should be considered more a friend than a foe of prostate cells, consistent with the observation that prostate glands from hypogonadal subjects are more inflamed than those from eugonadal ones. Interventional studies aimed at evaluating the anti-inflammatory effects of TRT in hypogonadal subjects with BPH are therefore warranted. Some pilot and RCT studies in hypogonadal men affected by BPH with or without MetS have documented the efficacy of TRT in reducing LUTS.41 In a small study on hypogonadal men affected by both BPH and MetS (n=95), normalization of plasma T levels, following treatment with T undecanoate, not only significantly improved MetS parameters, but also reduced the International Prostate Symptom Score (IPSS) and lowered the residual bladder urine volume, without any significant change in the prostate size.41 Shigehara and Namiki,41 in another small RCT with T enanthate or placebo in 46 men with mild BPH, showed a significant decrease in the IPSS, and amelioration in urodynamic parameters in the TRT group but not in the control one. Kalinchenko et al reported similar results.41 Some uncontrolled studies have reported a gradual improvement in the IPSS following long-term T therapy in men with hypogonadism and/or MetS.41 A small RCT with T enanthate in 23 men with BPH tended to support these findings, with a significant decrease in the IPSS, maximum flow rate, and voided volume in the T group but not in the 23 untreated controls.41 Finally, in a recent retrospective study on 120 hypogonadal men who underwent TRT, the group of McVary demonstrated that TRT not only has a low risk of worsening LUTS but induces an improvement in symptom scores in many men, while PSA changes appear to be minor.42
Because several studies have shown that TRT in older hypogonadal men alters PSA, but often below the normal upper limit, in the presence of severe symptoms of lower urinary tract obstruction - as indicated by an IPSS of 19 or greater - there is a relative contraindication to T treatment, according to recent guidelines.6,10,12
Erythrocytosis is an increase in the total red cell mass secondary to any of a number of non-hematologic systemic disorders in response to a known stimulus (secondary polycythemia), in contrast to primary polycythemia (polycythemia vera). Erythrocytosis is the most frequent adverse event associated with T administration in clinical trials, as well as in clinical practice. T is an important regulator of red blood cell production through augmentation of erythropoietin and through a direct effect on the bone marrow, including on erythroid progenitors, ferrokinetics, and red cell precursor survival. Recently, suppression of circulating and hepatic hepcidin (an iron regulatory peptide) has been proposed as an additional mechanism of action of T on red cell mass.43 Fig. 4A and 4B show the relationships between endogenous T levels and hemoglobin or hematocrit in almost 500 subjects consulting our Andrology Unit for sexual dysfunction. In contrast to the curvilinear relationship between T and PSA (Fig. 3), the associations between T and hemoglobin and hematocrit are better described by a linear relationship (adjusted r=0.162 and adjusted r=0.163, both p<0.0001, n=491), suggesting that there is a continuum in the T-induced promotion of red cell mass. In a meta-analysis of 19 RCTs including 1,084 subjects, T-treated subjects were nearly 4 times as likely as those in the placebo arm to develop a hematocrit >50%.44 Similar results were reported by another meta-analysis.45 Erythrocytosis has been reported to occur in healthy older men mainly after parenteral and oral T administration, but usually not after transdermal T.1,2,6 Some reports indicate the occurrence of myocardial infarction or stroke associated with T-induced polycythemia, resulting from intermittent high-dose T administration.1,2,6 Hence, it is important to monitor the hematocrit at regular intervals, to avoid potentially serious adverse events.1,2,6 In addition, pre-existing erythrocytosis constitutes a risk factor for thrombosis in hypogonadal men.1,2,6 Guidelines indicate that a hematocrit >54% is an indication to stop TRT until the hematocrit decreases to a safe level.6,10,12 Phlebotomy can also be considered in the most severe cases.
Obstructive sleep apnea (OSA) is the most common category of sleep-disordered breathing. Cautionary statements about T therapy in OSA appear frequently in the T therapy literature and guidelines, despite lack of convincing evidence.6 Only one RCT study that addresses this issue has been published so far.46 In obese men with severe OSA, T supplementation with a relatively high dose of parenteral T undecanoate for 18 weeks mildly worsened sleep hypoxemia acutely (at 7 weeks), but not at a later time point (18 weeks).46 However, Snyder et al47 could not detect any change in the Respiratory Distress Index after 36 months of transdermal T. Hence, despite the warnings in the package inserts, it is evident that the link between T therapy and OSA is relatively weak.
Infertility, defined as the inability to conceive after 1 year of unprotected intercourse, affects approximately 10% to 15% of reproductive aged couples in developed countries, and, in the vast majority, it is idiopathic. Administration of androgens has been proposed for more than 20 years as a therapy for idiopathic male infertility. However, a meta-analysis of 9 RCTs, involving 1,025 patients, revealed no significant influence of androgens on the pregnancy rate.48 These results are not surprising because androgens have been, for two decades, actively investigated for potential use in the opposite condition, that is, male medical contraception. In fact, because T exerts negative feedback on the hypothalamus and pituitary, TRT may temporarily suppress the hypothalamic-pituitary-gonadal axis, if this axis was previously at least partly functional. Therefore, spermatogenesis, no longer stimulated by gonadotropins, becomes less efficient or even suppressed. Accordingly, cases of TRT-induced male infertility have been reported.49 This negative impact on fertility is transient and disappears some months after discontinuation of TRT. In cases in which fertility is desired, agents that stimulate the endogenous production of T such as human chorionic gonadotropin (HCG) or, in mild cases, anti-estrogens should be prescribed.2 On the other hand, in young men with hypogonadotropic hypogonadism, long-term TRT does not compromise the forthcoming responsiveness to LH and FSH when fertility is desired,2,50 and may even in some cases be followed by recovery of pulsatile secretion of gonadotropins with T secretion and spermatogenesis.51 However, a recent report indicates that prior androgen therapy is independently associated with a decrease in the likelihood of achieving conception.52
In conclusion, T treatment in healthy older men in near-physiological doses does not appear to incur serious adverse events, although long-term safety has not been established, and regular monitoring of PSA and hematocrit levels is required.
The EMAS study demonstrated that sexual symptoms, and in particular, ED, reduced frequency of sexual thoughts, and reduced nocturnal erections, were the most important symptoms related to LOH (see above).13,53 Accordingly, applying EMAS criteria, we found that the overall prevalence of LOH in our patients was significantly higher when compared to that reported in the original EMAS study (see above).13 Hence, patients seeking medical care for sexual dysfunction represent a population with a high proportion of LOH cases, and T evaluation should be suggested before prescribing any kind of treatment.1,2 In contrast to the general population, we recently reported that in subjects with sexual dysfunction, the presence of sexual symptoms does not help raise the suspicion of hypogonadism among clinicians, because of lack of specificity, in particular when a severe form of hypogonadism is considered.54,55 Other clinical signs, such as increased waist circumference, might help, but only the clustering of several symptoms and signs, as reported in specific structured interviews such as ANDROTEST, result in a reliable screener for this condition.55
T plays a crucial role in regulating male sexual response. The libido is profoundly regulated by circulating T levels. Accordingly, we recently reported that in patients seeking medical care for sexual dysfunction, more than 40% of those with hypogonadism (total T below 12 nmol/L) reported a form of reduced libido.56 In these subjects, the severity of reduced libido increased according to the T decline, further substantiating the concept that a low androgen level is a clear, level-dependent risk factor for secondary reduced libido.56 Conversely, the specific role of T in regulating arousal and erectile function has not been completely clarified. In fact, T regulates both the relaxation of the corpora cavernosa thorough a positive action on nitric oxide synthase (NOS) formation and negative controls on the activity of the RhoA/ROCK (ras homolog gene family member a - rho associated, coiled-coil containing protein kinase) pathway.7 Accordingly, Fig. 5 shows a clear stepwise relationship between T and objective parameters derived from penile doppler ultrasound in a large series of subjects seeking medical care at our unit for sexual dysfunction. However, it is important to recognize that T also positively regulates the expression of phosphodiesterase type 5 (PDE5).57,58 Hence, since T positively controls both the enzymatic steps necessary for initiation (positive effect on NOS and negative on RhoA/ROCK) and the end (positive effect on PDE5) of the erectile process, its net effect on erection ends up as modest overall.7,57,58 The main physiological action of T is therefore to adjust the erectile process in a timely manner as a function of sexual desire, therefore finalizing erections with sex.7,57,58 TRT is able to improve sexual function in hypogonadal patients (total T <12 nmol/L);7,59-61 however, it has been suggested that aging could play an important role in evaluating the effect of TRT on sexual function.60 The latter observation is not surprising. In fact, while hypogonadism can be the main cause of ED in younger patients, it is generally only one element of a multifactorial ED in older ones.6,60 Considering the androgen-dependency of PDE5 (see above), in the last decade it has been suggested that the combination of TRT and PDE5 inhibitors (PDE5i) can be used to improve PDE5i outcomes in hypogonadal men.62 Accordingly, current guidelines emphasize the concept that hypogonadism must be ruled out or, if present, adequately treated, before prescribing PDE5i.6 Despite this evidence, in a double-blind, parallel, placebo-controlled trial, involving 140 hypogonadal individuals, Spitzer et al63 were unable to document any additional effect on erection by T in men treated for ED with the PDE5i sildenafil. However, it should be recognized that in this trial, TRT was initiated after a sildenafil-alone run-in period characterized by the increase of T levels already within the normal range (about 12.0 nmol/L [345 ng/dl]) in line with previous reports.64 Hence, the failure to respond to T in terms of erection was to be expected.64 However, the real efficacy of the combination of TRT and PDE5i needs to be proven in larger placebo-controlled studies since available studies enrolled small heterogeneous cohorts of participants with questionable diagnoses in terms of ED and hypogonadism and the meta-analytic result of the available evidence did not support this approach.6
Finally, it should also be recognized that clinical and experimental data have documented that T is profoundly involved in the regulation of the male ejaculatory reflex acting both at central and peripheral levels.65-67 Accordingly, our data showed that while patients reporting premature ejaculation have a reduced risk of hypogonadism, those complaining of delayed ejaculation or ejaculation volume reduction have a 2.5-fold increased chance of being hypogonadal.65 Hence, in our view, low T should be suspected and analyzed in all subjects with delayed ejaculation.
The relationship between reduced T levels and CVD still represents a matter of speculation. Cross-sectional epidemiological studies clearly show a significant association between low T and CVD.68,69 Accordingly, in patients seeking medical care for sexual dysfunction at our unit, we have reported an inverse relationship between the predicted cardiovascular risk as assessed by the Progetto Cuore risk engine and T levels (Fig. 4C). However, available evidence does not prove that T itself deserves the credit, and it is too soon to recommend TRT to try to lower heart disease risks. In fact, it is also known that any serious health condition can lower T levels. Hence, it is not completely known whether reduced T levels in aging males might play a direct pathogenetic role in the stratification of cardiovascular risk or if CVD and hypogonadism are concomitant conditions both associated with the aging process. Furthermore, the possibility that suppressed T during chronic diseases represents a desired protective mechanism, which could turn off T-dependent functions (such as reproduction and physical labor) that are not desired when the physical condition is ailing, cannot be excluded. Hence, the existence of a low T syndrome has been hypothesized.14,70,71 In line with this view, three independent meta-analyses published, so far, demonstrated an association between hypogonadism and overall- and cardiovascular-mortality, but they failed to find any statistical association with incident cardiovascular events.72-74 In addition, it has also been suggested that the increased risk associated with low T could be limited to elderly subjects.74
The main problem regarding the effect of TRT on cardiovascular risk is the lack of evidence from clinical trials actually testing whether TRT in older men decreases the risk of heart disease and stroke or not. A recent observational study evaluating the mortality rate in a series of 1,031 T-treated, compared with untreated hypogonadal (total T≤8.7 nmol/L) male veterans found that men receiving TRT have a 39% decrease in mortality, when compared to their untreated counterparts.75 Similar results have been previously reported in diabetic subjects.76 Interestingly, by meta-analyzing these studies, we found that the lack of TRT doubled the risk of mortality (Fig. 6). However, these results should be interpreted cautiously because residual confounding may still be a source of bias, including the substantial risk of a primary selection bias due to the nonrandom assignment of T exposure.
Only a few studies have investigated the effect of TRT in subjects with known CVD. Through the meta-analysis of available evidence, we previously reported that TRT was positively associated with a significant increase in treadmill test duration and time to 1-mm ST segment depression.72 However, the number of patients studied and the observational time were too small to draw definitive conclusions. In addition, three previous meta-analyses found no significant difference between the T and placebo groups for all cardiovascular events, nor for each type of event, except an increase in having a hematocrit of over 50%, which was significantly more prevalent in the T group.77-79 The statistical power of available meta-analyses is far from being adequate, due to limited sample series and the short duration of observations. However, a more recent meta-analysis including 27 RCTs with 2,994, eligible elderly men for the first time claimed the possibility that TRT might increase the risk of cardiovascular-related events.80 It is important to recognize that this meta-analysis included a larger number of studies evaluating the effect of TRT in older frail and sick men when compared to the previous ones. As also recognized by the authors, older people may be most affected by any decrement to their already poor health, so hospitalization may represent a particularly significant event.
In conclusion, the specific benefit of TRT in major cardiovascular outcomes, including mortality, deserves more and larger studies. We speculate that in conditions of cardiovascular frailty, the low T is due to a pre-existing disease, and is only a marker of poor health. In other words, low T characterizes subjects that are more frail, because they are affected by overt or silent chronic diseases, and therefore more prone to fatal cardiovascular events. According to the view of the 'low T syndrome', low T is an adaptive response in order to be more resilient to an abnormal environment (e.g., obesity) or to a pathological condition (e.g., T2DM, CVD), which are very common in the elderly, to save energy (less physical and sexual activity) and to protect the species (low fertility). Similar adaptive mechanisms have been previously described for other hormonal axes. For example, a typical pattern of altered thyroid hormone metabolism, characterized by low T3 circulating levels, is described as 'the low T3 syndrome'. The possibility that the low T syndrome could protect against forthcoming cardiovascular events might be of importance when considering replacement treatment in LOH, a condition in which low T is often associated with comorbidities related to cardiovascular burden. If the low T syndrome is a naturally occurring protective mechanism, its treatment might be deleterious for overall and cardiovascular health. However, it is possible that hypogonadism, even when caused by a pre-existing disease, might contribute to its progression or induce other diseases, finally resulting in an increased risk of fatal events.
The American Heart Association estimates that about 5.7 million Americans have congestive heart failure (HF), about 2 percent of the population.81 The condition accounts for about a third of heart-related deaths. Lower T levels have been shown to be an independent risk factor for worse outcomes among men and women with HF. In particular, it has been speculated that low T levels may contribute to the anabolic/catabolic imbalance, a typical feature of advanced HF.68 Patients with HF experience an impaired exercise capacity and muscle fatigue, not necessarily related to the degree of myocardial dysfunction, which can benefit from TRT.68 Hence, low T might contribute to some clinical features of advanced HF such as reduced muscle mass, energy handling, fatigue dyspnea, and finally, cachexia.68 In line with this hypothesis, a recent meta-analysis of placebo-controlled trials found that TRT was associated with a significant improvement in exercise capacity (including the 6-minute walk test, incremental shuttle walk test, and peak oxygen consumption) when compared with a placebo.81 However, it should be recognized that only four studies including 198 subjects were identified.
A large body of evidence supports the association between low T and impaired fasting glucose and insulin resistance,82,83 T2DM, and MetS.68,84-88 Fig. 4D shows, for example, the inverse relationship between the homeostatic model assessment (HOMA) Index and T levels in patients seeking medical care for sexual dysfunction in our unit. The specific pathogenetic mechanisms linking hypogonadism with MetS and insulin resistance appear to be complex and often multi-directional. Visceral obesity can probably be considered a relevant cause of hypogonadism, but at the same time, hypogonadism could be a cause of obesity and insulin resistance, consequently establishing a vicious cycle.68,84-88 Accordingly, although some prospective studies have demonstrated that low T at baseline predicts the development of both T2DM and MetS, other evidence suggests that T2DM or MetS at baseline could predict the occurrence of low T at follow-up.68,82-84 Based on this epidemiological evidence, current guidelines suggest that patients with clinical conditions associated with insulin resistance (obesity, T2DM, and MetS) should be screened for hypogonadism.6,10,12
Several uncontrolled studies have shown that TRT could improve body composition and reduced fat mass.64,84-88 The International, multicenter, Post-Authorization Surveillance Study on long-acting-intramuscular T undecanoate conducted on 1,493 hypogonadal men showed that, after 9~12 months, waist circumference decreased from 100 to 96 cm and blood pressure and lipid parameters were altered in a favorable and significant manner.89 Another more recent open-label, single-center, cumulative, prospective registry study of 255 hypogonadal men (aged 33~69 years) showed that normalizing serum T to normal physiological levels produced a consistent loss of body weight (-16 kg), waist circumference (-9 cm), and BMI (-4 kg/m2) over the full 5 years of the study.90
Despite this evidence, unfortunately only a few RCTs have evaluated the impact of TRT in patients with MetS and T2DM. We recently performed a meta-analysis of existing studies, now summarized in Table 3. So far, six RCTs have specifically evaluated the effect of TRT on MetS, enrolling a total of 483 patients, with a mean follow-up of 57 weeks.14 When only patients with T2DM were evaluated, 5 RCTs were available, enrolling 263 patients, with a mean follow-up of 28 weeks.14 Similarly to what was reported in our previous meta-analyses, TRT was associated with a significant reduction in fasting glycemia, HOMA Index, triglyceride levels, and waist circumference in patients with MetS (Table 3). Accordingly, an improvement in fasting glycemia, HbA1c, and triglyceride levels was observed in subjects with T2DM (Table 3).
Weight loss is able to reduce insulin resistance, preventing the progression to overt diabetes.14 Lifestyle intervention is therefore the widely recognized, first-line approach of all patients with T2DM.14 Unfortunately, diet and behavioral therapies often ultimately fail. Based on this evidence, lifestyle modifications should be strongly encouraged in hypogonadal subjects with obesity, T2DM, and MetS.6,10,12 Accordingly, the analysis of the longitudinal data of the EMAS study demonstrated that weight loss was associated with a proportional increase - and weight gain with a proportional decrease - in total T and SHBG.91 In line with these data, by performing a meta-analysis of the available studies, we recently demonstrated that weight loss, however it is obtained, significantly reverts obesity-associated hypogonadotropic hypogonadism.15 TRT and lifestyle modifications can also be combined, and this is strongly recommended in obese individuals with LOH. Accordingly, a combined intervention might maximize improvement in insulin sensitivity, reducing liver steatosis and increasing free-fat mass. However, this strategy has been tested only in two placebo-controlled RCTs.92,93 In the first study, Heufelder et al,92 in a small RCT involving 16 subjects with newly diagnosed T2DM and MetS, demonstrated that the combination of TRT and lifestyle intervention leads to a greater therapeutic improvement of glycaemic control and reverses the MetS condition after 52 weeks of treatment. Similarly, an 18-week, placebo-controlled RCT in obese patients with OSA demonstrated that the combination of TRT and lifestyle modifications improved insulin sensitivity, ameliorated fatty liver, and increased muscle mass, in comparison to placebo and lifestyle modification alone.93 By meta-analyzing the results of these studies, we report here that the combination of TRT and lifestyle modifications is able to improve waist circumference, the HOMA Index, and the lipid profile (reducing triglycerides and increasing high density lipoprotein cholesterol levels) when compared to both placebo and lifestyle modifications alone (Fig. 7).
T plays a major role in regulating male bone health, mainly through its aromatization to E2.6,14 However, some reports have indicated a possible independent link between reduced T levels and the risk of bone fractures, suggesting a possible direct role of T in increasing bone health.6,14 In line with the latter evidence, two independent meta-analyses have shown a positive effect of TRT on bone mineral density.94,95 Conversely, insufficient data have been published to calculate the effect of TRT on the risk of bone fractures.96
The prevalence of low T levels in human immunodeficiency virus (HIV)-infected men ranges from 20% to 25%. In particular, reduced levels of T are closely related to weight loss, progression to AIDS, wasting syndrome, depression, and loss of muscle mass and exercise capacity.14 The mechanisms underlying the association between LOH and HIV infection remains, however, not completely understood and has been reviewed elsewhere.14 Current guidelines suggest that clinicians consider short-term T therapy as an adjunctive therapy in HIV-infected men with low T levels and weight loss to promote weight maintenance and gains in lean body mass and muscle strength.6,10,12 In line with these suggestions, in a meta-analysis of the available placebo-controlled RCT in hypogonadal HIV-positive men, we found that TRT significantly improved lean mass over a placebo (Table 3).14 Similarly, a recent systematic review of the efficacy and safety of pharmacologic agents for the treatment of depressive and psychotic disorders in patients with HIV infection concluded that TRT may be effective in subsyndromal depression.97 However, it should be recognized that the results of the these trials were heterogeneous and limited by small sample size. Hence, further information would be required regarding the long-term benefits and adverse effects of anabolic steroid use in HIV subjects.
T deficiency is a common finding in men undergoing dialysis, to a great extent a consequence of the failing kidney per se. On the basis of laboratory testing, recent reports estimate a 40~60% prevalence of hypogonadism in men undergoing dialysis.14 Although insufficient information exists regarding the prevalence of this syndrome in chronic kidney disease (CKD) patients not requiring dialysis, it seems proportional to the reduction in renal function. Accelerated aging (uremia-associated oxidative stress), malnutrition, other associated conditions such as T2DM, chronic inflammation, vascular disease, and hypertension, as well as different classes of drugs used in these conditions, can all contribute to the development of CKD-associated hypogonadism, acting both at central and peripheral levels. Despite this evidence, however, it is still unknown whether in humans hypogonadism has any effect on progression to end-stage renal disease (ESRD). Interestingly, similar to what has been observed for CVD, recent evidence indicates that reduced T levels in hemodialysis patients are associated with increased all-cause and cardiovascular mortality,14 but no association with cardiovascular events has been reported. Taken together, these results suggest that low T, even in CKD, may be considered a marker of poor general health status, negatively affecting prognosis, rather than a specific cardiovascular risk factor. Interestingly, by meta-analyzing two placebo controlled RCTs combining the effect of steroid therapy and erythropoietin in the treatment of ESRD anemia, we recently reported that TRT might improve erythropoietin outcomes (Table 3).14 However, it should be recognized that studies in this area are scarce, and thus information about the benefits and risks of T therapy in ESRD male patients is too limited to draw final conclusions.
Chronic obstructive pulmonary disease (COPD) is a widespread systemic disease with high morbidity rates. Advanced stages can be complicated by unintentional weight loss and muscle wasting, which may contribute to increased morbidity and mortality. Reversal of weight loss increases muscle strength and exercise capacity and improves survival.14 The prevalence of hypogonadism in men with COPD can range from 22% to 69% and has been associated with several other systemic manifestations including osteoporosis, depression, and muscle weakness. However, it is unclear whether TRT can improve outcomes in COPD or can delay its progression. Interestingly, by meta-analyzing the few available data, even in COPD, TRT seems to be able to improve lean body mass (Table 3).14 Similar to what has been observed for CKD, however, it is unclear whether TRT can improve outcomes in COPD or can delay its progression.
Glucocorticoid therapy, a common treatment modality in COPD, is a common acquired cause of hypogonadism acting both at peripheral (reduction of T response to HCG stimulation) and pituitary (reduced pituitary response to GnRH) levels.14 Limited data suggest that lean mass and lumbar bone minerals may improve with TRT in subjects chronically treated with glucocorticoids.14
Opioids are among the most prescribed analgesic drugs, but present several side effects including nausea, itching, constipation, and hypogonadism.14 Opioid-induced androgen deficiency in males (OPIAD) is usually ignored by pain physicians and rarely considered for treatment, despite its high frequency (almost 100%) and persistence. Strong inhibition of androgen production quickly follows the onset of sustained-action opioid use, whether the opioids are administered orally, transdermally, or intrathecally.14 Interestingly, limited studies have shown that TRT in OPIAD can induce a general improvement of chronic pain and patients' quality of life, an important clinical aspect of pain management.14
Almost 80 years after the first chemical synthesis of T, the understanding of T deficiency (male hypogonadism), its determinants, and its consequences is still in its infancy. Evidence-based data have confirmed the close association between LOH and relevant sexual symptoms, including ED and low libido. Nonetheless, long-term RCTs evaluating the effects of T supplementation in hypogonadal subjects with ED or hypoactive sexual desire disorder are still needed. LOH is frequently comorbid with almost all severe and/or chronic diseases. In hypogonadal subjects with associated chronic morbidities, available evidence suggests that TRT is able to improve central obesity (subjects with MetS) and glycometabolic control (patients with MetS and T2DM), as well as to increase lean body mass (HIV, COPD), along with insulin resistance (MetS) and peripheral oxygenation (CKD). However, it should be recognized that the number of RCTs is too limited to draw final conclusions. Longer and larger studies are advisable to better clarify the role of TRT in such chronic conditions. Even though we cannot say, as Brown-Séquard did, that T is the 'rejuvenating elixir', we at the present time are reassured that TRT is at least not dangerous.
Figures and Tables
 | Fig. 1Classification of male hypogonadism as a function of age onset and patient's phenotype. Schematic prevalence in male population is also shown. Size of ellipsis reflects on abscissa (log scale): age of onset and on ordinates (log scale): incidence (right axis) or female to male phenotype (left axis, arbitrary unit). Adapted from ref. # 7. HCG: human chorionic gonadotropin, VEOH: very early onset hypogonadism, i.e. starting during foetal life for absence of testosterone formation or activity (e.g complete androgen insensitivity or Morris' syndrome, blue ellipsis) or impaired secretion or activity of GnRH (e.g., Kallmann's syndrome or mutation in GPR54 and GnRH receptor, red ellipsis), EOH: early onset hypogonadism (i.e peri-pubertal onset, such as in Klinefelter's syndrome, green ellipsis), LOH: late onset hypogonadism, i.e. in adulthood or aging (brown ellipsis). |
 | Fig. 2Prevalence of hypogonadism according to the European Male Aging Study (EMAS) criteria (13) in Florentine subjects of the EMAS study (n=433) and in a consecutive series of (n=3,293) output-patients attending medical care for sexual dysfunction between 2000~2011 at our center (UNIFI study). |
 | Fig. 3PSA levels as a function of total testosterone deciles in a consecutive series of 1,715 patients attending our Unit seeking medical care for sexual dysfunction and apparently free from prostate diseases: history of prostatitis, BPH, or suspected prostate cancer as assessed by urological examination and/or an abnormal (tender, enlarged, or with suspect nodules) digital rectal examination of the prostate, and/or total PSA above 4 ng/ml, and/or previous prostatic surgery or radiotherapy, and/or using drugs altering prostate growth (testosterone, alpha-blockers, vitamin D, and antiandrogen drugs including GnRH analogues, bicalutamide, 5-alpha-reductase inhibitors, serenoa repens and mepartricin). ANOVA Tukey post hoc was p<0.005 for 1st decile vs. 2nd or higher. PSA: prostate-specific antigen, BPH: benign prostatic hyperplasia. |
 | Fig. 4Age adjusted (adj) relationship between testosterone levels and hematocrit (A), hemoglobin (B), predicated cardiovascular (CV) risk as detected by Progetto Cuore risk engine (C) and homeostatic model assessment (HOMA) index (D). Data were obtained from a consecutive series of patients attending our unit seeking medical care for sexual dysfunction. |
 | Fig. 5Age adjusted (adj) relationship between testosterone levels and penile doppler ultrasound parameters performed before (flaccid) and after (dynamic) prostaglandin-1 stimulation. Data were obtained from a consecutive series of 2,345 patients attending our unit seeking medical care for sexual dysfunction. PSV: peak systolic velocity. |
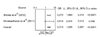 | Fig. 6Odds ratio (OR) for overall mortality in patients treated vs. not treated with testosterone replacement therapy (TRT). CI: confidence interval, LL; lower limit, UL: upper limit. |
 | Fig. 7Weighted differences (with 95% confidence interval [CI]) of waist circumference (A), homeostatic model assessment (HOMA) index (B), triglycerides (C) and high density lipoprotein (HDL) cholesterol (D) at endpoint across randomized controlled trials evaluating the combination of testosterone replacement therapy (TRT) and lifestyle modifications vs. lifestyle alone. The size of the circles reflects the sample dimension. LL: lower limit, UL: upper limit, Diff: difference. |
Table 2
Studies addressing the effect of testosterone replacement therapy in symptomatic hypogonadal men with prostate cancer not treated or after radical prostatectomy, brachytherapy, or external beam radiotherapy

References
1. Corona G, Rastrelli G, Forti G, Maggi M. Update in testosterone therapy for men. J Sex Med. 2011; 8:639–654.
2. Corona G, Rastrelli G, Vignozzi L, Maggi M. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2012; 17:239–259.

3. Andersson AM, Carlsen E, Petersen JH, Skakkebaek NE. Variation in levels of serum inhibin B, testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, and sex hormone-binding globulin in monthly samples from healthy men during a 17-month period: possible effects of seasons. J Clin Endocrinol Metab. 2003; 88:932–937.

4. Fellman J, Eriksson AW. Statistical analysis of the seasonal variation in demographic data. Hum Biol. 2000; 72:851–876.
5. Herndon JG, Perachio AA, Turner JJ, Collins DC. Fluctuations in testosterone levels of male rhesus monkeys during copulatory activity. Physiol Behav. 1981; 26:525–528.

6. Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013; 10:245–284.

7. Morelli A, Corona G, Filippi S, Ambrosini S, Forti G, Vignozzi L, et al. Which patients with sexual dysfunction are suitable for testosterone replacement therapy? J Endocrinol Invest. 2007; 30:880–888.

8. Mohr BA, Guay AT, O'Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf). 2005; 62:64–73.

9. Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008; 93:2737–2745.

10. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008; 159:507–514.

11. Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ. American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients--2002 update. Endocr Pract. 2002; 8:440–456.

12. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536–2559.

13. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010; 363:123–135.

14. Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism. Best Pract Res Clin Endocrinol Metob. 2013; [in press].
15. Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013; 168:829–843.

16. Corona G, Baldi E, Maggi M. Androgen regulation of prostate cancer: where are we now? J Endocrinol Invest. 2011; 34:232–243.

17. Corona G, Boddi V, Lotti F, Gacci M, Carini M, De Vita G, et al. The relationship of testosterone to prostate-specific antigen in men with sexual dysfunction. J Sex Med. 2010; 7:284–292.

18. Rastrelli G, Corona G, Vignozzi L, Maseroli E, Silverii A, Monami M, et al. Serum PSA as a predictor of testosterone deficiency. J Sex Med. 2013; [Epub ahead of print].

19. Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl. 2003; 24:299–311.

20. Kaufman JM, Graydon RJ. Androgen replacement after curative radical prostatectomy for prostate cancer in hypogonadal men. J Urol. 2004; 172:920–922.

21. Agarwal PK, Oefelein MG. Testosterone replacement therapy after primary treatment for prostate cancer. J Urol. 2005; 173:533–536.

22. Davila HH, Arison CN, Hall MK, Salup R, Lockhart JL, Carrion RE. Analysis of the psa response after testosterone supplemenatation in patients who have previously received management for their localized prostate cancer. J Urol. 2008; 179:Suppl 4. 428. abstract 1247.

23. Nabulsi O, Tal R, Gotto G, Narus J, Goldenberg L, Mulhall JP. Outcomes analysis of testosterone supplementation in hypogonadal men following radical prostatectomy. J Urol. 2008; 179:Suppl 4. 406. abstract 1244.

24. Khera M, Grober ED, Najari B, Colen JS, Mohamed O, Lamb DJ, et al. Testosterone replacement therapy following radical prostatectomy. J Sex Med. 2009; 6:1165–1170.

25. Leibowitz RL, Dorff TB, Tucker S, Symanowski J, Vogelzang NJ. Testosterone replacement in prostate cancer survivors with hypogonadal symptoms. BJU Int. 2010; 105:1397–1401.

26. Morales A, Black AM, Emerson LE. Testosterone administration to men with testosterone deficiency syndrome after external beam radiotherapy for localized prostate cancer: preliminary observations. BJU Int. 2009; 103:62–64.

27. Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer. 2007; 109:536–541.

28. Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D Jr, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol. 2011; 185:1256–1260.

29. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013; 25:24–28.

30. Fibbi B, Penna G, Morelli A, Adorini L, Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010; 33:475–488.

31. Vignozzi L, Gacci M, Cellai I, Morelli A, Maneschi E, Comeglio P, et al. PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate. 2013; [Epub ahead of print].

32. Vignozzi L, Gacci M, Cellai I, Santi R, Corona G, Morelli A, et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. 2013; 73:789–800.

33. Lotti F, Corona G, Degli Innocenti S, Filimberti E, Scognamiglio V, Vignozzi L, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013; 1:229–239.

34. Gacci M, Vignozzi L, Sebastianelli A, Salvi M, Giannessi C, De Nunzio C, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013; 16:101–106.

35. Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012; 212:71–84.

36. Morelli A, Comeglio P, Filippi S, Sarchielli E, Vignozzi L, Maneschi E, et al. Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: an experimental study in the rabbit. Prostate. 2013; 73:428–441.

37. Morelli A, Comeglio P, Filippi S, Sarchielli E, Cellai I, Vignozzi L, et al. Testosterone and farnesoid X receptor agonist INT-747 counteract high fat diet-induced bladder alterations in a rabbit model of metabolic syndrome. J Steroid Biochem Mol Biol. 2012; 132:80–92.

38. Vignozzi L, Morelli A, Filippi S, Comeglio P, Chavalmane AK, Marchetta M, et al. Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med. 2011; 8:57–77.

39. Filippi S, Vignozzi L, Morelli A, Chavalmane AK, Sarchielli E, Fibbi B, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009; 6:3274–3288.

40. Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. 2012; 214:31–43.

41. Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol. 2011; 52:657–663.

42. Pearl JA, Berhanu D, François N, Masson P, Zargaroff S, Cashy J, et al. Testosterone supplementation does not worsen lower urinary tract symptoms. J Urol. 2013; [Epub ahead of print].

43. Guo W, Bachman E, Li M, Roy CN, Blusztajn J, Wong S, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013; 12:280–291.

44. Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005; 60:1451–1457.

45. Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010; 95:2560–2575.
46. Hoyos CM, Killick R, Yee BJ, Grunstein RR, Liu PY. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2012; 77:599–607.

47. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999; 84:2647–2653.

48. Kamischke A, Nieschlag E. Analysis of medical treatment of male infertility. Hum Reprod. 1999; 14:Suppl 1. 1–23.

49. Bang JK, Lim JJ, Choi J, Won HJ, Yoon TK, Hong JY, et al. Reversible infertility associated with testosterone therapy for symptomatic hypogonadism in infertile couple. Yonsei Med J. 2013; 54:702–706.

50. Burger HG, de Kretser DM, Hudson B, Wilson JD. Effects of preceding androgen therapy on testicular response to human pituitary gonadotropin in hypogonadotropic hypogonadism: a study of three patients. Fertil Steril. 1981; 35:64–68.
51. Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007; 357:863–873.

52. Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009; 94:801–808.

53. O'Connor DB, Corona G, Forti G, Tajar A, Lee DM, Finn JD, et al. Assessment of sexual health in aging men in Europe: development and validation of the European Male Ageing Study sexual function questionnaire. J Sex Med. 2008; 5:1374–1385.
54. Corona G, Mannucci E, Ricca V, Lotti F, Boddi V, Bandini E, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl. 2009; 32:720–728.

55. Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. How to recognize late-onset hypogonadism in men with sexual dysfunction. Asian J Androl. 2012; 14:251–259.

56. Corona G, Rastrelli G, Ricca V, Jannini EA, Vignozzi L, Monami M, et al. Risk factors associated with primary and secondary reduced libido in male patients with sexual dysfunction. J Sex Med. 2013; 10:1074–1089.

57. Vignozzi L, Corona G, Petrone L, Filippi S, Morelli AM, Forti G, et al. Testosterone and sexual activity. J Endocrinol Invest. 2005; 28:3 Suppl. 39–44.
58. Corona G, Maggi M. The role of testosterone in erectile dysfunction. Nat Rev Urol. 2010; 7:46–56.

59. Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005; 63:381–389.

60. Boloña ER, Uraga MV, Haddad RM, Tracz MJ, Sideras K, Kennedy CC, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007; 82:20–28.

61. Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a metaanalysis. J Urol. 2000; 164:371–375.

62. Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, Fusco F. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med. 2011; 8:3418–3432.

63. Spitzer M, Basaria S, Travison TG, Bhasin S. In response. Effects of testosterone replacement on response to sildenafil citrate. Ann Intern Med. 2013; 158:570–571.
64. Aversa A, Jannini EA, Maggi M, Lenzi A. Effects of testosterone replacement on response to sildenafil citrate. Ann Intern Med. 2013; 158:569–570.

65. Corona G, Jannini EA, Lotti F, Boddi V, De Vita G, Forti G, et al. Premature and delayed ejaculation: two ends of a single continuum influenced by hormonal milieu. Int J Androl. 2011; 34:41–48.

66. Corona G, Jannini EA, Vignozzi L, Rastrelli G, Maggi M. The hormonal control of ejaculation. Nat Rev Urol. 2012; 9:508–519.

67. Corona G, Mannucci E, Petrone L, Fisher AD, Balercia G, De Scisciolo G, et al. Psychobiological correlates of delayed ejaculation in male patients with sexual dysfunctions. J Androl. 2006; 27:453–458.

68. Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab. 2011; 25:337–353.

69. Corona G, Monami M, Boddi V, Cameron-Smith M, Fisher AD, de Vita G, et al. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction. J Sex Med. 2010; 7:1557–1564.

70. Corona G, Rastrelli G, Monami M, Melani C, Balzi D, Sforza A, et al. Body mass index regulates hypogonadism-associated CV risk: results from a cohort of subjects with erectile dysfunction. J Sex Med. 2011; 8:2098–2105.

71. Corona G, Rastrelli G, Balercia G, Sforza A, Forti G, Maggi M. Testosterone and cardiovascular risk in patients with erectile dysfunction. J Endocrinol Invest. 2012; 35:809–816.
72. Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011; 165:687–701.

73. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011; 96:3007–3019.
74. Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011; 97:870–875.

75. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012; 97:2050–2058.

76. Muraleedharan V, Marsh H, Channer KS, Jones HT. Long-term testosterone replacement improves survival in men with type 2 diabetes and hypogonadism endocr rev. Endocr Rev. 2011; 32:OR35-2.
77. Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010; 95:2560–2575.
78. Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007; 82:29–39.

79. Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005; 60:1451–1457.
80. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013; 11:108.

81. Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012; 5:315–321.
82. Corona G, Rastrelli G, Balercia G, Lotti F, Sforza A, Monami M, et al. Hormonal association and sexual dysfunction in patients with impaired fasting glucose: a cross-sectional and longitudinal study. J Sex Med. 2012; 9:1669–1680.

83. Corona G, Rastrelli G, Silverii A, Monami M, Sforza A, Forti G, et al. The identification of prediabetes condition with ARIC algorithm predicts long-term CV events in patients with erectile dysfunction. J Sex Med. 2013; 10:1114–1123.

84. Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011; 34:557–567.
85. Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011; 34:528–540.

86. Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011; 8:272–283.

87. Corona G, Mannucci E, Forti G, Maggi M. Following the common association between testosterone deficiency and diabetes mellitus, can testosterone be regarded as a new therapy for diabetes? Int J Androl. 2009; 32:431–441.

88. Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011; 165:675–685.

89. Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, Maggi M. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med. 2013; 10:579–588.

90. Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring). 2013; [Epub ahead of print].

91. Camacho EM, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. EMAS Group. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013; 168:445–455.

92. Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009; 30:726–733.

93. Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012; 167:531–541.

94. Tracz MJ, Sideras K, Boloña ER, Haddad RM, Kennedy CC, Uraga MV, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006; 91:2011–2016.

95. Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005; 63:280–293.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download