Abstract
We present a rare case of a metastatic renal tumor originating from adenosquamous carcinoma of the intrahepatic bile duct. A 64-year-old man treated with bisegmentectomy and extended cholecystectomy for cholangiocarcinoma had a left cystic renal mass, which had irregular wall thickening, heterogeneously low attenuation, and soft tissue infiltration as determined by a computed tomography scan. The first impression was renal abscess. Left nephrectomy was performed and the nonencapsulated mass was gray in color macroscopically. Histological examination of the specimen revealed alveolar proliferation of small cancer cells, which was consistent with the original tumor of the intrahepatic bile duct. The left renal tumor was misdiagnosed as a renal abscess but finally diagnosed as squamous cell carcinoma metastasized from the intrahepatic bile duct. The patient expired because of lung metastasis after 14 months following left nephrectomy. In our opinion, this case would be the first report of a renal metastasis from a cholangiocarcinoma clinically and was treated with nephrectomy.
Renal metastases are common from disseminated malignancies, with a reported rate of 7.6~12% at autopsy. On the other hand, clinical detection of secondary carcinomas in the kidney are relatively rare.1-4 Although metastatic renal tumors are the usual findings, it is generally known that aggressive surgical treatment offers minimal survival benefit. Therefore, most cases have been managed conservatively.
We report a rare case of renal metastasis from carcinoma of the intrahepatic bile duct, misdiagnosed as a renal abcess. The patient survived about 14 months after nephrectomy.
A sixty-four-year old patient presented with complaints of fever, myalgia, and left flank pain lasting for one month. He had undergone bisegmentectomy and extended cholecystectomy for cholangiocarcinoma 110 days earlier and his symptoms had gradually been aggravated during close observation. On the pathologic report after the operation, more than one tumor larger than 5 cm was present and the cancer had spread to the regional lymph nodes. Thus his pathologic stage was T3N1M0.
The nature of his pain was gradual in onset and he had occasional nausea, minimal radiating pain nature and generalized weakness. There was history of fever without hematuria or pyuria. He was admitted our hospital via the emergency department. His vital signs were in the normal range, except body temperature, which was 38.1℃. A physical exam revealed costovertebral angle tenderness and a palpable left flank mass. Routine hematology and biochemical tests revealed leukocytosis (21,500 mm3), an elevated erythrocyte sedimentation rate (70 mm/hr), and c-reactive protein (13.7 mg/dl). Urinalysis and a chest radiograph were normal.
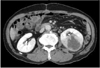
A computed tomography scan of the abdomen and pelvis showed a large left renal cystic lesion with soft tissue infiltration to the posterior perirenal fascia suggestive of pyonephrosis. The cyst had irregular wall thickening and heterogeneous attenuation (Fig. 1). The left kidney and ureter were normal. There was no ascites or lymphadenopathy. He had no history of past radiation exposure or renal stones.
We regarded this lesion as a renal abscess. To drain pus from the cyst, a percutaneous drainage tube was inserted under C-arm monitoring. However, the drained material was only blood, and no bacterial growth was found in the culture test. The mild fever persisted and flank pain was not controllable by analgesics. We suspected the cystic renal mass could be a malignant lesion. Therefore, we made a decision to perform nephrectomy for his uncomfortable symptoms and oncological management.
A extraperitoneal approach via the flank position was used. An approach to the kidney through the subcostal route was made and the perinephric space was entered by vertical incision to the Gerota fascia on the lateral aspect of the kidney, revealing underlying perinephric fat. By the guidance of a percutaneous drainage tube, we were able to reach the exact area of the tumor lesion. The kidney was mobilized sharply by developing a plane between the renal capsule and the perinephric fat.
Downward traction on the kidney permits the upper pole to be mobilized. With the use of lateral traction on the kidney to expose the hilum, the vascular pedicle is dissected free from the surrounding fat and lymphatics. The renal hilum can be approached anteriorly. The mobilized renal vein is retracted to reveal the renal artery located posteriorly. The left renal artery should be differentiated from the superior mesenteric artery by ensuring that the renal artery emanates from the lateral aspect of the aorta. The renal artery is ligated away from the hilum using a 2-0 silk tie. After division of the renal artery, the renal vein is similarly ligated and divided. In the posterior approach the artery is encountered before the renal vein and is ligated and the ureter is doubly clamped and divided. The specimen is removed. The distal aspect of the transected ureter is suture ligated. A drain is brought out through a separate stab incision.
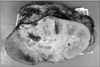
The specimen of the kidney measuring 12.2×7.0×5.2 cm in size and 440 g in weight was obtained. The whole mass was a distended sac-like structure without any grossly visible renal tissue. On section, the specimen showed an infiltrative solid mass along the lateral border. The cut surface showed a loculus with necrotic material within it (Fig. 2).
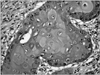
The histology of the cystic mass revealed features of moderately differentiated squamous cell carcinoma. Nests of infiltrating squamous cells with hyperchromatic nuclei and prominent keratin production were noted (Fig. 3). There were no positive findings on the periodic acid-Schiff stain. The tumor had invaded the renal parenchyma and perirenal soft tissue. There were no tumor emboli in the renal artery or veins. The entire tumor showed exclusive squamous differentiation. No transitional element was found within the tumor.
After nephrectomy, the palpable left flank mass with intermittent pain disappeared and the temperature returned to the normal range. During the follow up, general weakness with lung metastasis and multiple systemic dissemination were observed about 10 months after nephrectomy, and the patient expired 14 months after the nephrectomy.
Metastatic tumors in the kidney are common. Autopsy studies have shown that 7.6~12% of patients dying of cancer have renal metastases, making the kidney one of the most common sites for metastatic spreading.1-4 In general, these metastatic tumors are discovered at autopsy, so only 40 cases have been reported in studies that diagnosed them before the patient's death.5
In general, the high blood flow and profuse vascularity of the kidney make it an abundant growth medium for the deposition and growth of cancer cells. Most renal metastases develop through a hematogenous route of spread; only a small minority are caused by direct invasion of tumors derived from adjacent organs such as the pancreas, colon, and adrenal gland.
Primary tumors of the lung (19.8%), breast (12.3%) and stomach (11.1%) are the most common sources of renal metastases.6 Other sources include the cervix, prostate, gallbladder, thyroid, ovary, testis, urinary bladder, contralateral kidney, and bone.3
Choyke et al7 found that renal masses in patients with other known primary tumors were 4 times as likely to be metastatic than a primary renal tumor.5 Renal metastases are generally small, bilateral, and multifocal. However, there are no specific radiologic findings to distinguish a secondary renal tumor from primary renal cell carcinoma.4
In our case, a computed tomography scan showed a large renal cystic lesion highly suggestive of pyonephrosis. Therefore, percutaneous tube drainage with percutaneous fine-needle aspiration cytology could have been an appropriate next step for diagnosis and treatment in our situation. However, this confusion brought about the delay of palliative and therapeutic decision making on the nephrectomy in the end. We are not sure if nephrectomy should be more prompt.
Intrahepatic cholangiocarcinoma is an uncommon neoplasm and complete resection is the treatment of choice. If completely resected, 3-year survival rates range from 16% to 61% and 5-year survival rates, 24% to 44%. Factors associated with poor outcome include intrahepatic metastases, lymph node metastasis, vascular invasion, and positive margins. There is little evidence for the utility of radiation and chemotherapy in intrahepatic cholangiocarcinoma, and their use is optional.
In this case, the patient expired relatively earlier than we expected. At first, a renal mass biopsy or aspiration could be considered because it may preclude the need for surgical intervention.8 However, we can suspect that delay of nephrectomy resulted in shortening of the survival period. Though we had comparatively good control of the original tumor and also of the metastatic renal tumor by nephrectomy and of the intractable cancer-related symptoms (fever, myalgia, and flank pain), in case of any condition suggesting renal metastasis, the metastatic tumor should be the first diagnosis and all the treatment strategies should be focused on cancer control, because benign disease is rarely related to a survival disadvantage and a cancer-related event can lead to a regrettable result.
Figures and Tables
Fig. 1
Left cystic renal mass with irregular wall thickening, heterogeneous attenuation, and soft tissue infiltration.
References
1. Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950. 3:74–85.
2. Bracken RB, Chica G, Johnson DE, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979. 72:806–807.
3. Mayer RJ. Rieselbach RE, Garnick MB, editors. Infiltrative and metastatic disease of the kidney. Cancer and the kidney. 1982. Philadelphia: Lea & Febiger;707.
4. Sánchez-Ortiz RF, Madsen LT, Bermejo CE, Wen S, Shen Y, Swanson DA, et al. A renal mass in the setting of a nonrenal malignancy: When is a renal tumor biopsy appropriate? Cancer. 2004. 101:2195–2201.
5. Peterson RO. Urologic pathology. 1992. Philadelphia: Lippincott;127.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download