Abstract
A 57-year-old Korean woman visited 37 months after initial surgery with fetal head sized pelvic mass. Initially, she underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and para-aortic lymph node biopsy. The initial pathological report showed uterine smooth muscle tumors of uncertain malignant potential (STUMP). She had not received adjuvant therapy. Transvaginal ultrasound revealed multiple solid masses in the pelvic cavity. Abdomino-pelvic computed tomography scan revealed heterogeneous lobulated rounding masses. Tumor markers were all within the normal range. She underwent explorative laparotomy. The pathological diagnosis was epitheloid leiomyosarcoma. Patients with STUMP should be counseled regarding the potential for recurrence as leiomyosarcoma, and may require closer surveillance than a yearly.
Uterine smooth muscle tumor of uncertain malignant potential (STUMP) represents a poorly defined subcategory of uterine smooth muscle tumors. Diagnosis of STUMP can be difficult for pathologists, and understanding of clinical behavior of these tumors is poor [1]. Histologically, uterine smooth muscle tumors (SMTs) are categorized as leiomyomas and leiomyosarcomas, based on the combination of histological parameters, such as mitotic activity, cytological atypia, and coagulative tumor cell necrosis (CTCN) [2]. We report on a case of leiomyosarcoma with lymphatic dissemination of retroperitoneal leiomatosis, from previous hysterectomy state for treatment of STUMP.
A 57-year-old Korean woman (gravida 1, para 1) visited 37 months after initial surgery with fetal head sized pelvic mass in September 2009. She had no other history of systemic disease or surgery and had follow-up loss after initial surgery. Initially, she underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and para-aortic lymph node biopsy in August 2006. She had not received adjuvant therapy. The initial pathological report showed STUMP, 14×12 cm sized leiomyoma with no CTCN, mild atypia and focal moderate to severe atypia, and focal high mitotic area (up to 30 mitosis/10 high power fields [HPFs]). Paraganglioma and reactive lymphoid hyperplasia were observed in the para-aortic lymph node (Fig. 1).
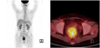
In September 2009, she was found a hard, palpable pelvic mass on pelvic examination. Transvaginal ultrasound revealed multiple solid masses measuring 4, 5, and 6 cm in the pelvic cavity. Abdomino-pelvic computed tomography (CT) scan revealed heterogeneous lobulated rounding masses measuring 4 × 4, 4 × 6, and 6 × 7 cm, which were suggestive recurrence of the pelvic cavity, and lymphatic dissemination of solid mass along iliac vessels (Fig. 2). F-18 fluorodeoxyglucose positron emission tomography (F-18 FDG PET)/CT scan showed several recurrent masses with abnormal FDG uptake in the pelvic cavity (Fig. 3). The serum level of cancer antigen (CA) 125, CA 19-9, and carcinoembryonic antigen were in normal range. Therefore, explolative laparotomy was performed. The surgical findings were as follows: no abnormal findings of the intra-abdominal cavity, multiple myoma along iliac artery, and a protruding mass under the rectal serosa. We performed complete removal of multiple myoma and retroperitoneal masses. On gross examination, the retroperitoneal masses were well circumscribed solid mass with grayish homogeneous cut surface. The frozen resection analysis reported leiomyoma and leiomyosarcoma, respectively. However, the final histopathological results showed that epitheloid leiomyosarcoma based on the immunohistochemical (IHC) staining: inhibin, cytokeratin, CD10, CD56, CD99, actin, and desmin. Results of IHC staining were positive for CD56, CD99, actin, and desmin. Mitotic activity was >30 mitosis count per 10 HPFs and evidence of CTCN was observed (Fig. 4). Additional IHC staining was performed for Ki-67, estrogen receptor (ER), and progesterone receptor (PR). The Ki-67 labeling index was approximately 10%, and results of IHC staining were positive for ER and negative for PR.
She underwent three cycles of chemotherapy with adriamycin (body surface area [BSA]×50 mg), ifosfamide (BSA×5 g), and mesna (BSA×5 g) on day 1, and cisplatin (BSA×50 mg) on day 2 every three weeks. Unfortunately, she passed away.
SMTs of the uterus remain a relatively uncommon diagnosis. SMTs range from benign leiomyomata to low- and high-grade leiomyosarcoma. A relatively rare variant of SMT is called STUMP and presents a dilemma for physicians due to its uncertain clinical behavior [3]. However, diagnosis of STUMP should be used most sparingly and every effort should be made to classify a smooth muscle tumor into a specific category [4].
The major histopathological parameters for assessing the diagnosis and prognosis of SMTs are cytological atypia, mitotic index, and CTCN. Based on these parameters, Bell et al. [5] subdivided STUMP into three histologically distinct groups with different clinical behaviors. One, the so-called "atypical leiomyoma with low risk of recurrence," is characterized by diffuse, moderate to severe cytological atypia, 10 MFs/10 HPFs, and no CTCN. Another type, called "atypical leiomyoma but experience limited," contains focal, severe cytological atypia, 20 MFs/10 HPFs, and no CTCN. The third group, called "smooth muscle tumors of low malignant potential," contains CTCN, 10 MFs/10 HPFs, and mild to absent cytological atypia.
In our case, the initial histopathologic finding was characterized by smooth muscle tumor with high celluarity not accompanied by CTCN or significant nuclear atypia. However, histopathologic finding of recurrent tumor showed of poorly differentiated cells with frequent mitotic figures and CTCN. Tumor cell necrosis is a diagnostically important finding that is present in 80% of leiomyosarcoma [5,6].
Very few studies have analyzed STUMPs with recurrences. Different histological classifications (not always using the Stanford criteria), diagnostic methods, length of follow-up and lack of detailed histological information make it difficult to compare the findings and draw conclusions. Guntupalli et al. [1] reported three patients (7.3%) had a recurrence during the follow-up period, one patient presented with a pelvic mass and a pulmonary nodule, and two patients presented with retroperitoneal and pelvic masses. Berretta et al. [2] presented a report of 3 cases with STUMPs. One patient developed diffuse lung metastases 9 years after the original diagnosis. Amant et al. [7] reported a retroperitoneal/pelvic relapse after 4 years in a patient diagnosed with a STUMP and treated with hysterectomy and adnexectomy. Previous studies suggest that STUMPs are usually clinically benign, but they should be considered as tumors of low malignant potential because they can occasionally recur or metastasize to distant sites, years after hysterectomy [8]. Even though all the reported cases of recurrent STUMPs survived (follow-up ranging from post-operative status to 157 months following the initial diagnosis), most of the results from the literature are controversial. There seems to be no consensus as to which histological features of STUMPs predict a higher probability of recurrence, the location of recurrence (sites reported include pelvis, abdomen, liver, lungs, lymph nodes, humerus, retroperitoneum, and uterus-if hysterectomy not performed), time to recurrence (between 15 months to 9 years), and histological type of recurrences (STUMP or leiomyosarcoma) [1,5,7,8]. There are no demographic characteristics to suggest predictive of recurrences [1]. In our case, patient experienced a retroperitoneal recurrence of leiomyosarcoma and lymphatic dissemination of retroperitoneal leiomatosis after 37 months diagnosed STUMP.
A few studies have identified IHC markers as a predictor of recurrences. Poorer prognosis is associated with the presence of p16 and p53 IHC positivity [8,9]. The IHC, including Ki-67, ER, and PR, is useful to distinguish between cases of malignant uterine SMTs and those of uncertain or borderline histology [10]. Overexpression of Ki-67 labeling index is frequently associated with leiomyosarcoma. In our case Ki-67 was about 10%, ER was positive, and PR was negative.
This is the report of a case of STUMP reteroperitoneal recurrence of leiomyosarcoma and lymphatic dissemination of retroperitoneal leiomatosis. STUMPs are low incidence disease and have an unpredictable clinical course. Patients with STUMP may require closer surveillance than a yearly. Further studies need to know malignant behavior from previous STUMP or de novo from retroperitoneal leiomyomatosis.
Figures and Tables
Fig. 1
(A) The tumor was characterized by smooth muscle tumor with high celluarity not accompanied by tumor cell necrosis or significant nuclear atypia (H&E, ×100). (B) However, a minor focal area exhibited moderate to severe nuclear atypia with high mitotic figures (H&E, ×200).
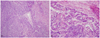
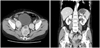
Fig. 2
Abdominopelvic computed tomography shows 4 × 4, 4 × 6, 6 × 7 cm sized heterogeneous lobulated rounding masses, suggestive recurrence of pelvic cavity, and lymphatic dissemination of rounding masses along iliac vessels.
References
1. Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009. 113:324–326.
2. Berretta R, Rolla M, Merisio C, Giordano G, Nardelli GB. Uterine smooth muscle tumor of uncertain malignant potential: a three-case report. Int J Gynecol Cancer. 2008. 18:1121–1126.
3. Shapiro A, Ferenczy A, Turcotte R, Bruchim I, Gotlieb WH. Uterine smooth-muscle tumor of uncertain malignant potential metastasizing to the humerus as a high-grade leiomyosarcoma. Gynecol Oncol. 2004. 94:818–820.
4. D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010. 116:131–139.
5. Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994. 18:535–558.
6. Hart WR. Problematic uterine smooth muscle neoplasms. Am J Surg Pathol. 1997. 21:252–255.
7. Amant F, Moerman P, Vergote I. Report of an unusual problematic uterine smooth muscle neoplasm, emphasizing the prognostic importance of coagulative tumor cell necrosis. Int J Gynecol Cancer. 2005. 15:1210–1212.
8. Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009. 33:992–1005.
9. Atkins KA, Arronte N, Darus CJ, Rice LW. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008. 32:98–102.
10. Slik K, El Rishi F, Bulugma M. Uterine smooth muscle tumors: clinicopathological evaluation. Free communication oral presentations. Int J Gynecol Obstet. 2009. 107(S2):S393–S396.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download