Abstract
Uterine rupture during the course of pregnancy is an uncommon obstetric event. Rupture of an unscarred uterus is rare, whereas rupture of a scarred uterus is more common and is usually the result of a Cesarean section. Uterine rupture during pregnancy usually occurs in patient with a scarred uterus, and recurrent uterine rupture usually occurs at a prior ruptured site. However, recurrent uterine rupture that occurs at a different site, regardless of prior rupture site, has yet to be reported. The patient described in this case report had cervical dysplasia with positive for human papilloma virus (HPV) type 16. As the presence of a precancerous lesion in the cervix is reported to increase the risk of preterm birth and spontaneous uterine rupture, a possible correlation between recurrent uterine rupture and cervical dysplasia was considered for this patient.
Uterine rupture during the course of pregnancy is an uncommon obstetric event. Rupture of an unscarred uterus is rare, whereas rupture of a scarred uterus is more common and is usually the result of a Cesarean section [1,2]. The uterus may also be scarred because of a previous perforation, hysterectomy, uteroplasty, cornual resection, or myomectomy (including laparoscopic) [3]. A scarred uterus is at high risk for uterine rupture during gestation and labor. Mortality rates strongly depend on both the time that elapses between onset and diagnosis of the uterine rupture, as well as the likelihood of immediate surgical intervention. Uterine rupture during pregnancy usually occurs after attempts at labor in patients with a scarred uterus, and recurrent uterine rupture usually occurs at a prior ruptured site. However, recurrent uterine rupture that occurs at a different site, regardless of prior rupture site, has yet to be reported.
The patient described in this case report had cervical dysplasia with positive for human papilloma virus (HPV) type 16. As the presence of a precancerous lesion in the cervix is reported to increase the risk of preterm birth and spontaneous uterine rupture [4], a possible correlation between uterine rupture and cervical dysplasia was considered for this patient. Here, we report a case of recurrent uterine rupture, with the second rupture located at a different site from the first rupture, in the third trimester of pregnancy in a patient with history of laparoscopic cornual resection and diagnosis of cervical dysplasia.
A 31-year-old primigravidarum visited our hospital for prenatal care at 6 weeks of gestation. She had a previous history of laparoscopic left cornual resection due to a left cornual pregnancy 2 years prior. On her initial prenatal evaluation, an ultrasound revealed no abnormal uterine structures. Cervical erosion was observed during a routine pelvic examination. A cervical biopsy was positive for HPV type 16, but a Pap test showed normal cervical epithelium without intraepithelial lesion or malignancy. She was admitted to our hospital due to abdominal discomfort at 31 weeks 5 days of gestation. During the physical examination, the patient was cooperative but suffered from upper abdominal discomfort. Measurements included a blood pressure of 100/60 mm Hg, a heart rate of 80 beats/min, and a body temperature of 36.8℃. Upon examination of the abdomen, old laparoscopy scars were observed on the skin. Upper abdominal tenderness, but not rebound tenderness, was noted. External fetal heart rate monitoring showed a normal variability pattern with a baseline of 140-150 beats/min. External monitoring of uterine activity revealed one contraction every 2 to 3 minutes. A speculum examination revealed that the cervix was normally positioned. A digital examination revealed a cervical ostial opening of 1.5 cm with 50% effacement. The initial hemoglobin level was 12.6 g/dL and the hematocrit was 35.2%. An abdominal ultrasound revealed a normal fetus with biometric measurements of 31 gestational weeks, normal amniotic volume, and a normal-appearing placenta. Under a diagnosis of preterm labor, ritodrine hydrochloride was administered intravenously. After hospitalization for 2 days, she complained of severe pain in the upper left quadrant, and her appearance showed acute illness. The pain worsened and radiated to her left shoulder. At that moment, her blood pressure was 90/60 mm Hg, and her heart rate was 100-110 beats/min. Both diffuse and rebound tenderness in the upper left quadrant were noted. Cardiotocographic monitoring was performed, revealing no signs of fetal distress. An ultrasound revealed a uterine wall defect on the left upper portion of the uterus and an extruded amniotic sac with fetal buttocks (Fig.1). Laboratory tests demonstrated dropping hemoglobin (9.5 g/dL) and hematocrit (26.5%) levels. The patient was considered to have an acute abdomen with suspicions of uterine rupture. Cesarean section with repair of the uterine rupture was performed immediately by the obstetrics team. The rupture site was 7 cm in diameter and located on the left cornual area of the uterus. The amniotic sac containing 200 mL hemorrhagic fluid was intact and protruded into the pelvic cavity through the defect. With the Cesarean delivery, a male baby weighing 1,530 g with Apgar scores of 3 at 1 minute and 6 at 5 minutes was born. We used the three-layer method to repair the defect. Initial horizontal suturing of the endometrial layer and part of the myometrium using 1-0 chromic catgut was performed followed by the use of locking sutures in the myometrium to close the defect; finally, the serosa was closed using continuous inverting sutures composed of 3-0 chromic catgut. The patient was given antibiotics and had a good postoperative recovery. She was discharged 5 days later. The newborn baby was moved to the general ward from the neonatal intensive care unit after 1 month without any complications.
The patient visited our hospital for routine postnatal care and cervical cancer screening after delivery of her first baby. The patient underwent several Pap tests that revealed atypical squamous cells of undetermined significance.
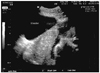
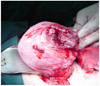
The patient became pregnant again the following year and received regular prenatal care on nine different occasions. At 33 weeks 3 days of gestation, the patient was hospitalized due to acute, severe pain in the right upper abdomen. On admission, cyclic uterine contractions were observed by non stress test in addition to continuous severe abdominal pain with local rebound tenderness. Tocolysis was induced by the intravenous infusion of ritodrine. Measurements included a blood pressure of 110/80 mm Hg, a heart rate of beats/min, and a body temperature of 36.8℃. Her vaginal discharge was white, and the uterine cervical os was dilated to one finger's width. Laboratory analysis revealed a hemoglobin level of 10.1 g/dL, a hematocrit of 29.5%, and a platelet count of 157,000/µL. Results of biochemical and coagulation tests were all normal. An abdominal ultrasound revealed a thin uterine wall with a minor fetal part beneath it (Fig. 2). Ultrasound findings led us to suspect uterine rupture, and an emergent exploratory laparotomy was performed. A section (300 mL) of the hemoperitoneum was removed, and a bulging amniotic sac was noted. An approximate 7 cm of the rupture site was located on the upper posterior uterine wall (Fig. 3). An additional transverse incision was made to this site, and a 2,604 g male baby was delivered with Apgar scores of 6 and 8 at 1 and 5 minutes, respectively. The estimated blood loss during the operation was 700 mL. We excised the thin part of the uterine wall and reconstructed the site. The patient was discharged uneventfully 5 days after surgery.
The reported incidence of uterine rupture ranges from 1 in 8,000 to 1 in 15,000 pregnancies [5,6]. Because the key factor in pregnant uterine rupture is a scarred uterus, and most importantly, uterine rupture often occurs during the intrapartum, careful monitoring of uterine activity and a high degree of suspicion of uterine rupture makes early diagnosis possible [7]. Most uterine ruptures have various risk factors. The single most important factor for uterine rupture is a previous scar. Scars from Cesarean delivery, hysteroscopic resection of a uterine septum, myomectomy, and cornual resection are all considered causes of uterine rupture. Such ruptures may occur in patients with parity, placenta increta or percreta, adenomyosis, abortion with instrumentation, manipulation during delivery, misoprostol-induced delivery, vigorous fundal pressure during delivery, or cocaine abuse. There are little data regarding the risk of uterine rupture and the optimal route of delivery for future pregnancies after a laparoscopic treatment for an interstitial pregnancy [8]. Uterine rupture during subsequent pregnancies is a well-known complication of operative laparoscopy and, invariably, with extraction of the fetus during the second or third trimester [9,10].
The likelihood that a uterine scar will rupture during a subsequent pregnancy depends strongly on scar location [11]. The overall risk of rupture of corporal scars varies from 4% to 19% [12]. Comparing lower uterine segment and corporal scars, the latter ruptures more easily, tends to rupture prior to the onset of labor, and represents a more serious complication. However, the second rupture site was not the same as the first rupture site in this case.
An HPV DNA test performed prior to the first pregnancy was positive for HPV type 16, and atypical squamous cells of undetermined significance following the first uterine rupture. In addition, invasive squamous cell carcinoma was diagnosed from a uterine cervical punch biopsy carried out after the second uterine rupture. There is increasing evidence that the presence of a precancerous lesion (even without treatment) is associated with an increased risk of preterm birth and spontaneous uterine rupture [13]. Several studies have reported that benign conditions such as infection and congenital cervical anomalies or malignant conditions such as cervical cancer cause secretions from and gradual enlargement of the uterus, leading to thinned uterine walls that may be sloughed off with spontaneous uterine rupture and cause generalized peritonitis [14,15]. Thus, these observations together suggest that precancerous or malignant conditions of the uterine cervix cause the uterus to become thinner, thereby increasing risk of preterm labor as well as vulnerability for rupture at a site different from the previous scar. The results of this case also suggest that ultrasound may be useful for the detection of this condition, even in the absence of typical symptoms of uterine rupture, such as hypovolemic shock and fetal distress. Herein, an accurate diagnosis was made by ultrasound and proper management was performed immediately.
In conclusion, clinicians should consider recurrent uterine rupture as a possible diagnosis when patients present with abdominal pain and have a history of laparotomy or laparoscopy and/or benign or malignant conditions, even without typical symptoms of a ruptured uterus.
Figures and Tables
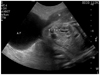 | Fig. 1Uterine wall defect and extruded amniotic sac with the fetal buttocks during the 1st uterine rupture. AF, amniotic fluid; M, myometrium; P, placenta. |
References
1. Al-Zirqi I, Stray-Pedersen B, Forsén L, Vangen S. Uterine rupture after previous caesarean section. BJOG. 2010. 117:809–820.
2. Kaczmarczyk M, Sparén P, Terry P, Cnattingius S. Risk factors for uterine rupture and neonatal consequences of uterine rupture: a population-based study of successive pregnancies in Sweden. BJOG. 2007. 114:1208–1214.
3. Turner MJ. Uterine rupture. Best Pract Res Clin Obstet Gynaecol. 2002. 16:69–79.
4. Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG. 2011. 118:1031–1041.
5. van Alphen M, van Vugt JM, Hummel P, van Geijn HP. Recurrent uterine rupture diagnosed by ultrasound. Ultrasound Obstet Gynecol. 1995. 5:419–421.
6. Berghahn L, Christensen D, Droste S. Uterine rupture during second-trimester abortion associated with misoprostol. Obstet Gynecol. 2001. 98:976–977.
7. Mishra A, Landzberg BR, Parente JT. Uterine rupture in association with alkaloidal ("crack") cocaine abuse. Am J Obstet Gynecol. 1995. 173:243–244.
8. Su CF, Tsai HJ, Chen GD, Shih YT, Lee MS. Uterine rupture at scar of prior laparoscopic cornuostomy after vaginal delivery of a full-term healthy infant. J Obstet Gynaecol Res. 2008. 34:688–691.
9. Pelosi MA 3rd, Pelosi MA. Spontaneous uterine rupture at thirty-three weeks subsequent to previous superficial laparoscopic myomectomy. Am J Obstet Gynecol. 1997. 177:1547–1549.
10. Hockstein S. Spontaneous uterine rupture in the early third trimester after laparoscopically assisted myomectomy. A case report. J Reprod Med. 2000. 45:139–141.
11. Lantinen AJ. Labor following previous cesarean section. Am J Obstet Gynecol. 1980. 137:517.
12. Spaulding LB, Gallup DG. Current concepts of management of rupture of the gravid uterus. Obstet Gynecol. 1979. 54:437–441.
13. Lee SL, Huang LW, Seow KM, Hwang JL. Spontaneous perforation of a pyometra in a postmenopausal woman with untreated cervical cancer and "forgotten" intrauterine device. Taiwan J Obstet Gynecol. 2007. 46:439–441.
14. Chan LY, Yu VS, Ho LC, Lok YH, Hui SK. Spontaneous uterine perforation of pyometra. A report of three cases. J Reprod Med. 2000. 45:857–860.
15. Vyas S, Kumar A, Prakash M, Kapoor R, Kumar P, Khandelwal N. Spontaneous perforation of pyometra in a cervical cancer patient: a case report and literature review. Cancer Imaging. 2009. 9:12–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download