Abstract
Mesotheliomas are rare neoplasms of the pleura, peritoneum or pericardium, which are frequently associated with exposure to asbestos. Early diagnosis and establishment of a standard treatment for malignant peritoneal mesothelioma is difficult. We report on a case of malignant peritoneal mesothelioma in a 53-year-old woman with no history of exposure to asbestos or previous radiotherapy.
In the United States, mesothelioma, a rare cancer, is estimated to occur in approximately 2,500 people annually [1]. Sporadic cases have been reported in Korea. Although, malignant pleural mesothelioma is the most common type of mesothelioma, mesothelioma has also observed in the peritoneum and pericardium. Malignant pleural mesothelioma is observed mainly in older men (median age, 72 years) who have been exposed to asbestos, although it occurs decades after exposure (20 to 40 years later) [2]. Most cases of mesothelioma have been linked to asbestos exposure, however, some reports have suggested that radiotherapy may also be a cause of mesothelioma [3,4]. Reports of malignant peritoneal mesotheliomas in women are rare. The significant variation in incidence may reflect erroneous diagnosis of peritoneal serous carcinomas or well-differentiated papillary mesotheliomas as malignant peritoneal mesothelioma. Frequency of exposure of asbestos in patients with malignant peritoneal mesothelioma ranges from 0 to 50% [5]. Malignant peritoneal mesotheliomas are most commonly diagnosed during the fifth to seventh decades of life [6].
We report on a case of malignant peritoneal mesothelioma in a 53-year-old woman with no history of exposure to asbestos or previous radiotherapy, along with a brief review of the literature.
A 53-year-old woman was transferred to our hospital in September 2010, complaining of fever and right flank pain. She was in good health and other than being a housewife, she had no history of employment. Abdomino-pelvic computed tomography (CT) revealed a lobulated contoured heterogeneously enhancing mass, which measured 7.5 × 5.2 × 9.5 cm, in the right subhepatic space and multiple small nodular lesions with fatty infiltration in mesentery and omentum (Fig. 1). 18-fluorodeoxyglucose positron emission tomography (PET)-CT showed diffuse increased metabolic activity in peritoneum and mesentery of the right subhepatic space (Fig. 2). Results of pelvic ultrasonogram, gastrofiberscopy, and colonoscopy were nonspecific. Cancer antigen (CA) 125 was 867 U/mL (normal range, <35.0 U/mL), however, carcinoembryonic antigen (CEA) and CA 19-9 were within the normal range. Based on these findings, we had the impression of peritoneal carcinomatosis. The patient underwent explorative laparotomy, so that an optimal debulking operation could be performed for confirmation of the diagnosis. The tumor consisted of a firm, bulky white mass that involved the greater omentum, right parietal peritoneum, and greater curve of the stomach. Numerous white nodules, which varied in diameter, were observed along the mesentery of the small intestine and throughout the abdominal cavity. Yellowish ascites were also observed. Frozen biopsy of the right subhepatic mass indicated poorly differentiated carcinoma. Cytoreductive surgery (total abdominal hysterectomy, bilateral salphingo-oophorectomy, omentectomy, parietal peritonectomy, and diaphragm peritonectomy) was performed to the greatest extent possible; however, the operation was suboptimal. The diaphragm near the margin of the liver and the peritoneum on the posterior vertebral area could not be removed. The final pathologic finding was malignant peritoneal mesothelioma, biphasic type with a positive resection margin. Microscopic finding of the mass revealed solid sheet-like arrangement of malignant epithelial mesothelial cells and short fascicles of spindle shaped malignant mesothelial cells. Vesicular nuclei, macronucleoli, and abundant glassy eosinophilic cytoplasm resembling a decidual reaction were observed in epithelial mesothelial cells. Frequent mitotic figures and necrosis were also observed (Fig. 3). Immunohistochemical study of the tumor showed positivity for cytokeratin, calretinin, and vimentin, but was negative for diastase resistant periodic acid-Schiff, Wilm's tumor-1 protein (WT-1), CEA, and cluster of differentiation 15 (Fig. 4).
After surgery, we planned chemotherapy with administration of a cisplatin (80 mg/m2)/pemetrexed (500 mg/m2) regimen every three weeks. The patient repeated chemotherapy with eight cycles. However, two months after undergoing chemotherapy, she had recurrence. Second-line chemotherapy with paclitaxel (175 mg/m2) and carboplatin (area under curve ×5) was attempted, but yielded a poor response. Despite the change of chemotherapy regimens, her general condition became aggravated. The patient is currently under hospice care, and is being treated as a terminal cancer patient.
Malignant peritoneal mesothelioma is an aggressive neoplasm, which arises from serous membranes, such as the pleura, pericardium, and peritoneum. Due to restriction of asbestos since the 1970s, incidence of malignant peritoneal mesothelioma has leveled off in the United States, however, incidence in other countries, including Western Europe and Australia, is still on the rise [7,8].
Patients with malignant peritoneal mesothelioma most commonly present with nonspecific abdominal symptoms, such as abdominal pain, distension, weight loss, and ascites, resulting in diagnosis when the condition is relatively advanced. An abdominal or pelvic mass is often palpable [6].
Grossly, both the parietal and visceral peritoneal surfaces are studded with nodules or plaques of tumor that vary in size. Diffuse thickening of the peritoneum or omentum may also be observed. As the tumor spreads, it may encase the viscera and infiltrate the bowel wall. The tumor may, on occasion, be metastasized to the retroperitoneum, including the pancreas, lymph nodes, liver, lung, and pleura [6,9]. Malignant peritoneal mesothelioma is composed of two major histotypes, epithelioid (most common) and sarcomatoid, with the biphasic being a mixture of both. In more detail, several subtypes or patterns of differentiation are recognized, particularly for the epithelioid histotype. The most frequently occurring epithelioid patterns are tubulo-papillary, adenomatoid (microglandular), and sheet-like, while less common patterns include deciduoid, small cell, or clear cell. Reports of pleomorphic and lympho-histiocytoid subtypes are rare [10]. Mesothelial cells are derived from coelomic epithelium via mesenchymal-epithelial transition (MET), which explains the co-expression of cytokeratin and vimentin [10,11]. This pattern of co-expression is retained in the malignant mesothelioma. Of particular importance, transdifferentiation from epithelioid to sarcomatoid histo-type may be considered as epithelial-mesenchymal transition, reflecting the histotype plasticity of this neoplasm. During this reverse process, in comparison with MET, expression of cytokeratins, calretinin and podoplanin (D2-40) is often reduced, whereas expresstion of vimentin remains stable or is increased [10].
Malignant peritoneal mesotheliomas are classified as biphasic when both the epithelial and stromal components are clearly malignant. Although the two components may be intimately intermixed, they more commonly form separate areas of the neoplasm [9]. The presence of columnar cells, overlapping nuclei, presence of slit-like spaces, numerous psammoma bodies and intracytoplasmic epithelial mucin are features that favor a diagnosis of peritoneal serous adenocarcinoma over malignant peritoneal mesothelioma [11].
Special stains, electromicroscopy and immunohistochemical stains are valuable for differentiation of epithelial malignant peritoneal mesothelioma from peritoneal serous adenocarinoma. Immunohistochemical staining is the tool used most often to aid in distinguishing malignant peritoneal mesothelioma from peritoneal serous adenocarcinoma. Vimentin is regarded as the primary marker for MET. Immunohistochemical studies for markers such as calretinin and keratin 5/6 facilitate recognition of the mesothelial nature of this neoplasm. Malignant peritoneal mesothelioma is characterized by positive staining for the following immunohistochemical markers: epithelial membrane antigen, calretinin, WT-1, cytokeratin 5/6, antimesothelial cell antibody-1, hector bacttifora mesothelial cell-1, mesothelin, and thrombomodulin [12,13]. Usefulness of serum markers CA 125 and CA 153 in diagnosis and monitoring of peritoneal mesothelioma has recently been reported [13]. Soluble mesothelin-related protein (SMRP) and osteopontin are prospective serum markers for diagnosis and follow-up. Elevated levels of SMRP are observed in more than 71% of mesotheliomas [13].
There is not yet a consensus with regard to the optimal treatment. The overall rate of response to systemic chemotherapy with pemetrexed/cisplatin has been reported as approximately 25% and a median overall survival of approximately 1 year. Recent studies on the molecular biology of malignant peritoneal mesothelioma have yielded new insights relating to the potentially important role of the phosphatidylinositol 3-kinase/mammalian target of rapamycin and epidermal growth factor receptor pathways in this disease, which may translate into new therapeutic options for patients with malignant peritoneal mesothelioma [14]. In selected patients, an association of operative cytoreduction and hyperthermic intraoperative peritoneal chemotherapy using cisplatin or mitomycin C with long-term survival has been reported [15].
The prognosis of malignant peritoneal mesothelioma is very poor, with a median survival of only about one year. In the hope of developing an effective multimodality therapy, we report on a case of a malignant peritoneal mesothelioma. Further study may be needed in order to provide evidence based regimens for diagnosis and treatment.
Figures and Tables
Fig. 1
Abdomino-pelvic computed tomography revealed 7.5 × 5.2 × 9.5 cm-sized lobulated contoured heterogeneously enhancing mass in right subhepatic space, multiple small nodular lesions with fatty infiltration in mesentery and omentum.

Fig. 2
(A,B) Positron emission tomography-computed tomography showed diffuse increased metabolic activity in peritoneum and mesentery of right subhepatic space.
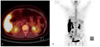
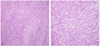
Fig. 3
(A) The tumor revealed solid sheet-like arrangement of malignant epithelial mesothelial cells and short fascicles of spindle shaped malignant mesothelial cells. Epithelial mesothelial cells showed vesicular nuclei, macronucleoli, and abundant glassy eosinophilic cytoplasm resembling a decidual reaction (H&E, ×100). (B) Frequent mitotic figures and necrosis are also found (H&E, ×200).
References
1. Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol. 2009. 27:2081–2090.
2. Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992. 34:718–721.
3. Teta MJ, Lau E, Sceurman BK, Wagner ME. Therapeutic radiation for lymphoma: risk of malignant mesothelioma. Cancer. 2007. 109:1432–1438.
4. De Bruin ML, Burgers JA, Baas P, van't Veer MB, Noordijk EM, Louwman MW, et al. Malignant mesothelioma after radiation treatment for Hodgkin lymphoma. Blood. 2009. 113:3679–3681.
5. Plaus WJ. Peritoneal mesothelioma. Arch Surg. 1988. 123:763–766.
6. Asensio JA, Goldblatt P, Thomford NR. Primary malignant peritoneal mesothelioma. A report of seven cases and a review of the literature. Arch Surg. 1990. 125:1477–1481.
7. Larson T, Melnikova N, Davis SI, Jamison P. Incidence and descriptive epidemiology of mesothelioma in the United States, 1999-2002. Int J Occup Environ Health. 2007. 13:398–403.
8. Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer. 1999. 79:666–672.
9. McCaughey WT, Kannerstein M, Churg J. Armed Forces Institute of Pathology (US). Universities Associated for Research and Education in Pathology. Tumors and pseudotumors as the serous membranes. 1985. Washington, DC: Armed Forces Institutes of Pathology.
10. Soltermann A, Pache JC, Vogt P. Metastasis of a pleural mesothelioma to a hyperplastic stomach polyp: an increase of vimentin expression is seen during a gain in deciduoid morphology. Rare Tumors. 2011. 3:e52.
11. Truong LD, Maccato ML, Awalt H, Cagle PT, Schwartz MR, Kaplan AL. Serous surface carcinoma of the peritoneum: a clinicopathologic study of 22 cases. Hum Pathol. 1990. 21:99–110.
12. Marchevsky AM. Application of immunohistochemistry to the diagnosis of malignant mesothelioma. Arch Pathol Lab Med. 2008. 132:397–401.
13. Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008. 9:147–157.
14. Turner K, Varghese S, Alexander HR Jr. Current concepts in the evaluation and treatment of patients with diffuse malignant peritoneal mesothelioma. J Natl Compr Canc Netw. 2012. 10:49–57.
15. Golse N, Bakrin N, Passot G, Mohamed F, Vaudoyer D, Gilly FN, et al. Iterative procedures combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal recurrence: postoperative and long-term results. J Surg Oncol. 2012. 106:197–203.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download