Abstract
Krukenberg tumor is not easy to detect during pregnancy. One reason is its rare incidence because of the chronological gap between the reproductive age and the high risk age for gastric cancer. Another reason is the similarity between the typical symptoms of normal pregnancy and the Krukenberg tumor: epigastric discomfort, early satiety, nausea, etc. We report a case of a pregnant woman in her third trimester who presented with chronic back pain and sudden paraplegia.
A young pregnant woman who had a history of chronic back pain came to our medical center. On the day she came, she had experienced sudden paralysis of both legs.
During the Cesarean section she was found to have a 7 cm sized adnexal mass, and an advanced staged Krukenberg tumor with bone metastasis was diagnosed later.
Despite all efforts, the patient expired two months after the delivery. Only early detection, followed by aggressive surgery and chemotherapy can be expected to yield a better outcome.
A 26-year-old woman, gravida 1 para 0, came to us with paralyzed legs. This unmarried patient was in her 28th week and 6th day of her gestation and had undergone little antepartum care. She had a history of chronic back pain and a 2-month-long period of vomiting with early satiety. Despite the gradually aggravated symptoms, she never sought proper medical attention. Instead, she would visit an Oriental medical clinic for temporary pain relief. On the day she came to us, she had experienced sudden paralysis of both legs. At first, she visited a branch of our medical center and underwent magnetic resonance imaging there, which revealed an L2 bursting fracture. The fracture was suggested as the cause of the paralysis (Fig. 1), although she had not experienced any previous traumatic event. She was referred to our hospital for further evaluation.
Her back pain had rapidly aggravated and was hard to control. The Neurosurgery Department concluded a surgical decompression to relieve both the pain and the paralysis. The operation required that she remain in a prone position for hours, which could harm the fetus. In addition, the bleeding expected during the operation could also jeopardize the baby. Therefore, we decided to perform an elective Cesarean section first for fetal safety. The patient was at the 30th week of the gestation by then.
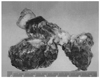
Preoperatively, abdominal ultrasonography revealed ascites in the perihepatic and perisplenic regions, both paracolic gutters, and Morison's pouch. Mild splenomegaly was also noted. She bore a 1.42 kg preterm male baby with Apgar scores of 6 and 7 at 1 and 5 minutes after birth. During the section, she was found to have a friable right adnexal mass measuring 7 cm at its greatest diameter (Fig. 2), which the sonographer missed preoperatively, and diffuse extensive gastric wall thickening was noted from the fundus to the antrum. An ovarian frozen biopsy revealed malignancy, so adnexectomy was performed immediately. At the same time, we noticed a hard omental mass and adhesion in the upper peritoneal cavity, especially around the stomach. The General Surgery team could not execute a co-operation due to the thick adhesion and the hard omental cake around the stomach. Postoperatively, multiple metastases to the bones, including spine, shoulder, pelvic bone, femur, and tibia, were identified by a bone scan.
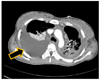
After the diagnosis of an advanced staged Krukenberg tumor, whose pathologic type was poorly differentiated metastatic adenocarcinoma, the planned decompressing neurosurgery was canceled. Instead, chemotherapy was scheduled for as soon as her general condition improved. However, she developed dyspnea and tachycardia, with a heart rate up to 190 bpm. A postoperative chest computed tomography (CT) on the 5th day after operation suggested a bilateral large pleural effusion with pneumonia. This was followed by a high fever. Due to a large amount of malignant pleural effusion (Fig. 3), which was later revealed by transudate and negative for malignant cell, chest tubal drainage was needed on the 6th postoperative day. A blood culture showed Candida and Acinetobacter on the 12th postoperative day, suggesting her poor condition. Acute respiratory distress syndrome and repeated respiratory arrests developed. Despite all efforts, the patient expired two months after the delivery.
Compared to other malignancies, Krukenberg tumor has several specific characteristics. One of these is the nature of the presenting symptoms, which usually include vague abdominal pain, distention, and ascites. Nearly 90% of patients have these symptoms [1]. The diagnosis of this tumor in pregnancy is often overlooked due to the similarities of the signs and symptoms of pregnancy. The gastrointestinal symptoms that mimic the early nausea and vomiting of pregnancy may create difficulties in diagnosing a tumor in the stomach. Furthermore, the expanding pregnant uterus may mask the abdominal distension caused by the ovarian tumor. This was true in our case. The patient presented several symptoms simultaneously. Diagnosis could be facilitated by the clinical symptoms, which hinted at multiple organ involvement. Another peculiar feature of Krukenberg tumor is the mean age of its occurrence. Women are typically diagnosed with Krukenberg tumors in the fifth decade of life, meaning that most patients are in the premenopausal period of life. This fact can be one of the reasons of the rarity of Krukenberg tumor. With respect to our case, the development of a Krukenberg tumor in pregnancy is much rarer, occurring at a rate of approximately 1 case in every 100,000 pregnancies [2]. Another reason why it is so rare is the gap between the child-bearing period and the high risk period in life. About 6% of malignant ovarian tumors are metastatic in origin and Krukenberg tumors account for 1% to 2% of all ovarian neoplasia worldwide [2]. They are encountered in younger patients more often than are other metastatic ovarian neoplasms.
The route of metastasis of gastric carcinoma to the ovaries has long been a mystery. Generally, the spread of metastases may occur via the lymphatics, the blood, or through both routes. Invasion into the lymphatic system is followed by the transport of tumor cells to regional nodes and ultimately to other parts of the body. This is the most common route of metastasis for carcinomas. On the other hand, hematogenous spread is typical of all sarcomas and is the favored route in certain carcinomas. Because of their thinner walls, veins are more frequently invaded than are arteries, and metastasis follows the pattern of the venous flows. For the Krukenberg tumor, early lymphatic invasion, followed by subsequent spread into the systemic circulation, is suggested to be an important metastatic pathway. Consequently, the relatively earlier age at diagnosis is hypothesized to be related to the great vascularity of the ovaries, which facilitates vascular metastasis. Although a counterargument is that the ovaries are spared from intraperitoneal adhesions and present without any peritoneal deposits, Woodruff and Novak reported some cases of Krukenberg tumors with ascites at the time of diagnosis, suggesting that peritoneal spread may be another route of metastasis [1]. Reports of bone metastasis of Krukenberg tumor are rare. Zeigerman described a case of primary Krukenberg tumor with osteoplastic metastases predominantly to axial skeleton [3]. Kiyokawa et al. reported an extensive review of 120 cases in which two-thirds of the Krukenberg tumors were diagnosed at the same time as the primary carcinoma [4]. Diagnosis is usually accomplished by CT scan or ultrasound, but the identification of the primary site of the Krukenberg ovarian tumors is often quite challenging. Thus, the diagnosis requires a high index of suspicion and careful assessment.
The cells in a metastatic tumor resemble those in the primary tumor and a tendency exists for certain tumors to seed in particular organs. This was first discussed as the "seed and soil" theory by Stephen Paget in 1889 [5]. Krukenberg tumor is a good example of this theory. According to the theory, the survival of cancer cells is difficult outside their region of origin; consequently, in order to metastasize, they must find a location with similar characteristics. For example, breast tumor cells, which gather calcium ions from breast milk, metastasize to bone tissue, where they can gather calcium ions from bone. According to "seed and soil" theory, tumor seeds spread, via the lymphatics, the circulation, or other routes, to the whole body. However, the seeds only germinate in a proper location with similar characteristics to those of the original tumor. In the case of Krukenberg tumors, this 'fertile soil' can be the breast, lung, bone, lymph nodes, skin, etc. Bone metastases of gastric cancer are rare, but if they are diagnosed, patients survive only 2 to 5 months [3,6].
No effective treatment has yet been established for Krukenberg tumor other than complete surgical resection of both the primary and metastatic lesion. Nevertheless, surgical resection is only effective in patients with limited metastasis that is confined to the ovaries. In our case, multiple bone metastases had already developed prior to her admission, so palliative care was the only option possible for her.
It is also interesting to note that some surgeons recommend prophylactic oophorectomy as part of the surgical procedure for gastric cancer patients. Only early detection, followed by aggressive surgery and chemotherapy, can be expected to yield a better prognosis. A high suspicion must be present in order not to diagnose Krukenberg tumors too late.
Figures and Tables
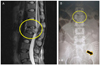 | Fig. 1(A) Preoperative finding. Magnetic resonance imaging shows bone metastasis of Krukenberg tumor resulting in L2 bursting fracture (circle) followed by sudden paraplegia. (B) Preoperative finding. L-spine anterior posterior view shows 30+0 week-aged fetus (arrow) in uterus and L2 spinal fracture (circle) simultaneously. |
References
1. Dueñas-García OF, Diaz-Sotomayor M, Chanana C. Bilateral ovarian krukenberg tumor in a full-term pregnancy. ISRN Obstet Gynecol. 2011. 2011:620380.
2. Mandai M, Konishi I, Tsuruta Y, Suginami N, Kusakari T, Iwasaki T, et al. Krukenberg tumor from an occult appendiceal adenocarcinoid: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2001. 97:90–95.
3. Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the 'seed and soil' theory of metastatic dissemination. Clin Exp Med. 2006. 6:145–149.
4. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006. 30:277–299.
5. Finlay M, Sherman C, Rubenstein J, Wierzbicki R, Chow E. A late relapse of primary Krukenberg tumour with bone metastases. Clin Oncol (R Coll Radiol). 2003. 15:500–503.
6. Jain D, Mahajan N, Singh T. Metastatic signet ring cell adenocarcinoma of bone marrow with bilateral ovarian masses: a case report. Cases J. 2008. 1:332.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download