Abstract
Objective
Methods
Results
Figures and Tables
 | Fig. 1Study design. Nine-week-old male and female Spraque-Dawley rats were used in this study. After a 1-week acclimation period, rats were mated. At day 10 of gestation, rats were randomly divided into two groups and given an ad libitum (AdLib) standard laboratory chow or 50% food restricted diet determined by quantification of normal intake in ad libitum fed rats until delivery. Offspring were divided randomly into four groups: 1) AdLib/AdLib, given ad libitum diet throughout experimental period; 2) food restricted (FR)/AdLib, given 50% diet during pregnancy and ad libitum diet during lactation and adulthood; 3) AdLib/FR, given ad libitum diet during pregnancy and 50% FR diet during lactation and adulthood; and 4) FR/FR, given 50% FR diet throughout experimental period. |
 | Fig. 2Body weight of male and female offspring in AdLib/AdLib, AdLib/FR, FR/AdLib, and FR/FR groups during experimental period. (A) Body weight in male offspring and (B) body weight in female offspring. AdLib/AdLib, given ad libitum diet throughout experimental period; FR/AdLib, given 50% food restricted (FR) diet during pregnancy and ad libitum diet during lactation and adulthood; AdLib/FR, given ad libitum diet during pregnancy and 50% FR diet during lactation and adulthood; FR/FR, given 50% FR diet throughout experimental period. |
Table 1

Values are presented as mean ± standard error of the mean values.
AdLib/AdLib, given ad libitum diet throughout experimental period; FR/AdLib, given 50% food restricted diet during pregnancy and ad libitum diet during lactation and adulthood; AdLib/FR, given ad libitum diet during pregnancy and 50% food restricted diet during lactation and adulthood; FR/FR, given 50% food restricted diet throughout experimental period; NS, no significant.
Table 2
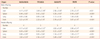
Values are presented as mean ± standard error of the mean values.
AdLib/AdLib, given ad libitum diet throughout experimental period; FR/AdLib, given 50% food restricted diet during pregnancy and ad libitum diet during lactation and adulthood; AdLib/FR, given ad libitum diet during pregnancy and 50% food restricted diet during lactation and adulthood; FR/FR, given 50% food restricted diet throughout experimental period; NS, no significant.
a,b,cDifferent superscript letters indicate the comparison with significant differences; dRelative organ weight (g/100 g body weight).
Table 3

Values are presented as mean ± standard error of the mean values.
AdLib/AdLib, given ad libitum diet throughout experimental period; FR/AdLib, given 50% food restricted diet during pregnancy and ad libitum diet during lactation and adulthood; AdLib/FR, given ad libitum diet during pregnancy and 50% food restricted diet during lactation and adulthood; FR/FR, given 50% food restricted diet throughout experimental period; NS, no significant.
a,b,cDifferent superscript letters indicate the comparison with significant differences; dRelative organ weight (g/100 g body weight).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download