Abstract
Peripheral primitive neuroectodermal tumor (PNET) is a small round tumor belonging to the PNET/Ewing's sarcoma family classified based on location in the body. There have been a small number of case reports of PNET arising in the ovary. We present extremely rare case of PNET of the ovary occurring in a 32-year-old pregnant woman, which was detected during her second Cesarean section. She had a past history of oophorectomy due to a huge mature solid teratoma in left ovary during her first Cesarean section. She got the operation of right salpingoophorectomy during the second Cesarean section at local gynecologic clinic and referred to our institute. Magnetic resonance imaging and positron emission tomography revealed the PNET was metastased to the peritoneum and the lymph nodes. We had persecuted vincristine, doxorubicin, cyclophosphamide, and etoposide/ifosfamide 8 cycles. There has been no evidence of local tumor recurrence and metastasis after the chemotherapy until now.
Primitive neuroectodermal tumor (PNET), which is monodermal teratoma composed of neuroectodermal tissue, is the second most common sarcoma among children and young adult [1]. It may occur anywhere in the body and within any age group; however, it is most likely to occur in the bone and soft tissues [2]. PNET has been reported at a number of extraskeletal sites, the most common organ-based location is the kidney, but being other sites include ovaries, uterus, abdominal cavity, peritoneum, and lung [3,4]. There have been a small number of case reports of PNET arising in the ovary. Spontaneous pregnancy in a patient with PNET of the ovary after conservative fertility-preserving treatment have been previously reported [5]. This case represents the first case diagnosed as the PNET of the ovary at pregnancy women who diagnosed with mature solid teratoma at the opposite side of ovary.
A 32-year-old woman was referred to the Samsung Medical Center (SMC) with complaint of metastatic ovarian tumor which was detected during Cesarean section. She was in her usual state of health until she had oophorectomy 3 years ago during her previous Cesarean section due to mature solid teratoma of left ovary. Based on operation record she brought, about 8-cm sized of mass beside right ovary with ruptured and multiple peritoneal seeding masses were observed. Right salpingoophorectomy and peritoneal mass excisional biopsy were performed. No macroscopic residual disease was noted. On pathologic report, macroscopically, the enlarged right ovarian mass was encapsulated measuring 8.0×5.0×1.5 cm and suspected rupture. The most of cut surface was replace with mass, solid, friable, yellowish brown colored. The peritoneal nodal mass was encapsulated measuring 2.5×1.5×1.5 cm and characteristics were almost similar with solid ovarian mass.
She was admitted to the SMC for evaluate extent of metastasis and provide proper treatment of PNET of the ovary. On review of system and physical examination, there was no significant abnormality. Laboratory evaluations were all within normal range. Tumor marker study including CA-125, alpha-fetoprotein, and beta -human chorionic gonadotropin was within normal range. Histologically, the tumor cells were PNET in ovary (Fig. 1). The tumor was monodermal malignant tumor and had focal glial differentiation. The microscopic appearance of the specimen showed a tumor with undifferentiated, primitive-appearing, round blue cells forming sheets of Homer-Wright rosettes. On immunohistochemical stains, the tumor cells were strongly positive for vimentin, synaptophysin and MIC2 protein (CD99). The tumor cells were focaly positive by immunostaining to S-100 protein and glial fibrillary acidic protein (GFAP). For other markers such as neurofilament, cytokeratins (AE1/AE3 and cytokeratins 7, cytokeratins 20) and epithelial membrane antigen (EMA), observed negative in tumor cells.
Based on imaging, surgical, and pathology findings, the diagnosis was made of PNET of the ovary accompanied by peritoneal seeding. Metastatic work-up of this patient revealed peritoneal seeding and the lymph nodes of both internal iliac area detected by abdomen and pelvic magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) (Fig. 2). An abdomen and pelvic MRI showed peritoneal and omental thickening, loculated hematoma and fluid collection in the abdomen and pelvic cavity. The findings are likely suggestive of peritoneal seeding. A PET-CT showed faint, heterogeneous radiotracer uptake with a more intense focus superiorly in the region of bilateral internal iliac lymph node area. There were mild and diffuse fluorodeoxyglucose (FDG) uptakes in the pelvic cavity corresponding to omental thickening on abdomen and pelvic MRI.
Consultation with medical oncologists led to a decision to treat her with chemotherapy. She received multiple regimen chemotherapy with vincristine, doxorubicin, cyclophosphamide, and etoposide, ifosfamide (VDC/IE). The chemotherapy regimen consisted of vincristine 1.5 mg/m2 during 1 day (maximum 2 mg), doxorubicin 75 mg/m2 during 1 to 2 days, cyclophosphamide 1.2 g/m2 during 1 day, every 6 weeks. After 3 weeks, ifosfamide 1.8 g/m2, etoposide 100 mg/m2 all administered on during 1 to 5 days, every other 6 weeks. Human granulocyte colony-stimulating factor support 5 µg/kg/day by subcutaneous form during about 24 hours following completion of every cycles of chemotherapy. Every three cycles, CT scan of the abdomen and pelvis was performed. Response to initial chemotherapy was good. A CT scan of the abdomen and pelvis after only three cycles of therapy could not identify clear evidence of residual disease.
After total eighth courses of chemotherapy, CT scan of the abdomen and pelvis showed no evidence of metastatic disease to the abdomen and pelvic cavity. After completion of eight cycles the patient was in full clinical, biochemical, and radiographic remission. The following workup included a PET scan, repeat CT scan of the abdomen and pelvis, chest X-ray (PA), blood tests include complete blood cell count, chemistry, lactate dehydrogenase every 3 month until first 1 year, after than 6 months until 5 years after completion of chemotherapy. All of them revealed no evidence of metastatic disease and she remains in complete remission with no evidence of disease 54 months from presentation.
The reported incidence of adnexal masses in pregnancy ranges from 1 in 81 to 1 in 8,000 pregnancies. The overall incidence of malignancy including low-malignant potential tumors in an adnexal mass noted in pregnancy is 1%-8% [6-8]. There are only a few case reports of PNET arising from the ovary diagnosed during pregnancy [5,9].
PNET of the ovary is rare monophasic teratoma composed exclusively of neuroectodemal tissue. They are poorly differentiated (primitive) tumor group of neuroectodermal tumors divided for clinical-pathologic purposes [1]. Immunohistochemical, karyotypic, and reverse transcription-polymerase chain reaction analysis could diagnose the tumor.
Histologically, PNET of the ovary is all composed of primitive neuroblastic or primitive, developmentally uncommitted precursors, of neural and glial cells. PNET of the ovary is highly cellular and composed of small cells with hyperchromatic, round to oval nuclei and scanty cytoplasm. These cells are arranged into lobules separated by fibrovascular septa, but also may form pattern-less sheets. Varying amounts of finely fibrillar cell processes are present in the tumor. Areas of necrosis can be prominent [5,10].
Diagnosing PNET of the ovary is quite straight forward when tumor shows variable reactivity with antibodies to CD99, neuron-specific enolase and vimentin. Most cells are negative for, but scattered cells showing neural or glial differentiation will be positive for neurofilaments and synaptophysin, or GFAP and S-100. No cells react with antibodies for cytokeratin, desmin, chromogranin or inhibin [11,12].
Molecular genetic analysis can be an adjunct to the diagnosis of PNET of the ovary as it shows Ewing's sacroma gene/Friend leukemia virus integration 1 gene (EWS/FLI-1) fusion gene transcript type 2 by reverse transcriptase-polymerase chain reaction and EWS gene rearrangement by fluorescence in situ hybridization [13,14]. The results of comparative genomic hybridization revealed multiple chromosomal abnormalities in PNET of the ovary. RT-PCR analysis showed that N-myc and EGFR were overexpressed, while Rb and ARHI were underexpressed [15].
PNET of the ovary are very rare and aggressive tumors which are associated with high morbidity and mortality. Many of the larger studies of PNET of the ovary suggested that these tumors were highly aggressive neoplasms that rapidly give rise to metastatic disease and death. The survival rate varied from 10.8 months to 3 years. The prognosis for patients with PNET/Ewing's sarcoma family has steadily improved, as most patients with localized tumors are now cured by surgery, radiation therapy, multi-agent chemotherapy, or both. Chemotherapy using Vincristine, Doxorubicin, and cyclophosphamide (and more recently ifosfamide and etoposide as our patient) was used for Ewing's sarcoma.
In summary, peripheral PNET found in adult is an uncommon malignancy and, to our knowledge, there was no case of this tumor occurring in pregnant women who had diagnosed mature solid teratoma of ovary ever described. Further studies are necessary to assess the effect of multi-agent chemotherapy as optimal therapy for all patients with metastatic ovarian PNET.
Figures and Tables
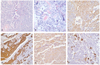 | Fig. 1Primitive neuroectodermal tumor of the ovary. The tumor is composed of nondescript small blue cells arranged in a patternless manner. The microscopic appearance of the specimen showed a tumor with undifferentiated, primitive-appearing, round blue cells forming sheets of Homer-Wright rosettes (A, B) A, H&E, ×200; B, H&E, ×400. Immunohistochemically, vimentin (C), synaptophysin (D), MIC2 protein (CD99) (E) are expressed within cytoplasms of tumor cells. And the larger tumor cells show focal glial fibrilar acid protein expression, which is suggestive of more differentiation from small primitive cells (F). (C-F, Immunohistochemical staining, ×400). |
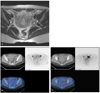 | Fig. 2Abdomen and pelvic magnetic resonance imaging (MRI) and positron emission tomography-computed tomography imaging. MRI shows two borderline sized lymph nodes in both internal iliac areas that cannot be excluded metastatic lymph node (A). Maximum diameter of right ovary was measured 3.3 cm and it has multiple physiologic cysts inside (B). Lymph node metastasis seem to be contributing to increasing fluorodeoxyglucose (FDG) uptake at left internal iliac lymph node (P-SUV=3.7) and both external iliac lymph nodes (P-SUV=Lt:5.0, Rt:4.6) (C). It detected soft tissue mass-like lesion increasing of FDG uptake around left anterior pelvis (P-SUV=6.5). It should consider chance of malignancy (D). |
References
1. Dehner LP. Primitive neuroectodermal tumor and Ewing's sarcoma. Am J Surg Pathol. 1993. 17:1–13.
2. Kim KJ, Jang BW, Lee SK, Kim BK, Nam SL. A case of peripheral primitive neuroectodermal tumor of the ovary. Int J Gynecol Cancer. 2004. 14:370–372.
3. Katz RL, Quezado M, Senderowicz AM, Villalba L, Laskin WB, Tsokos M. An intra-abdominal small round cell neoplasm with features of primitive neuroectodermal and desmoplastic round cell tumor and a EWS/FLI-1 fusion transcript. Hum Pathol. 1997. 28:502–509.
4. Marley EF, Liapis H, Humphrey PA, Nadler RB, Siegel CL, Zhu X, et al. Primitive neuroectodermal tumor of the kidney: another enigma: a pathologic, immunohistochemical, and molecular diagnostic study. Am J Surg Pathol. 1997. 21:354–359.
5. Demirtas E, Guven S, Guven ES, Baykal C, Ayhan A. Two successful spontaneous pregnancies in a patient with a primary primitive neuroectodermal tumor of the ovary. Fertil Steril. 2004. 81:679–681.
6. Hoover K, Jenkins TR. Evaluation and management of adnexal mass in pregnancy. Am J Obstet Gynecol. 2011. 205:97–102.
7. Leiserowitz GS. Managing ovarian masses during pregnancy. Obstet Gynecol Surv. 2006. 61:463–470.
8. Kumari I, Kaur S, Mohan H, Huria A. Adnexal masses in pregnancy: a 5-year review. Aust N Z J Obstet Gynaecol. 2006. 46:52–54.
9. Ateser G, Yildiz O, Leblebici C, Mandel NM, Unal F, Turna H, et al. Metastatic primitive neuroectodermal tumor of the ovary in pregnancy. Int J Gynecol Cancer. 2007. 17:266–269.
10. Morovic A, Damjanov I. Neuroectodermal ovarian tumors: a brief overview. Histol Histopathol. 2008. 23:765–771.
11. Takano T, Akahira J, Moriya T, Murakami T, Tanaka M, Goto M, et al. Primary ependymoma of the ovary: a case report and literature review. Int J Gynecol Cancer. 2005. 15:1138–1141.
12. Hirahara F, Yamanaka M, Miyagia E, Nakazawa T, Gorai I, Minaguchi H, et al. Pure ovarian ependymoma: report of a case treated with surgery, chemotherapy, irradiation and hyperthermotherapy. Eur J Obstet Gynecol Reprod Biol. 1997. 75:221–223.
13. Urano F, Umezawa A, Yabe H, Hong W, Yoshida K, Fujinaga K, et al. Molecular analysis of Ewing's sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Jpn J Cancer Res. 1998. 89:703–711.
14. Kawauchi S, Fukuda T, Miyamoto S, Yoshioka J, Shirahama S, Saito T, et al. Peripheral primitive neuroectodermal tumor of the ovary confirmed by CD99 immunostaining, karyotypic analysis, and RT-PCR for EWS/FLI-1 chimeric mRNA. Am J Surg Pathol. 1998. 22:1417–1422.
15. Chow SN, Lin MC, Shen J, Wang S, Jong YJ, Chien CH. Analysis of chromosome abnormalities by comparative genomic hybridization in malignant peripheral primitive neuroectodermal tumor of the ovary. Gynecol Oncol. 2004. 92:752–760.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download