Abstract
Gestational trophoblastic neoplasm includes tumor spectrum of four entities: hydatidiform mole (complete and partial), invasive mole, choriocarcinoma and placental site trophoblastic tumor. The hydatidiform mole is usually benign, but it is regarded as a pre-malignant disease. The other three conditions are malignant and are termed gestational trophoblastic tumor. Although most molar pregnancies behave in a benign fashion, metastatic tumors develop after complete molar pregnancy in 4% of patients. However, even when the disease is spread to many distal organs, it is highly curable with chemotherapy in most cases. We recently encountered an unusual case of metastatic gestational trophoblastic neoplasm following complete mole, presenting as spontaneous renal and cerebral hemorrhage with a fatal course.
Go to : 
References
2. Martin BH, Kim JH. Changes in gestational trophoblastic tumors over four decades. A Korean experience. J Reprod Med. 1998; 43:60–8.
3. Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010; 203:531–9.

4. Crawford RA, Newlands E, Rustin GJ, Holden L, A'Hern R, Bag-shawe KD. Gestational trophoblastic disease with liver metastases: the Charing Cross experience. Br J Obstet Gynaecol. 1997; 104:105–9.
5. Carlini L, Villa A, Busci L, Trezzi G, Agazzi R, Frigerio L. Selective uterine artery embolization: a new therapeutic approach in a patient with low-risk gestational trophoblastic disease. Am J Obstet Gynecol. 2006; 195:314–5.

6. Pearl ML, Braga CA. Percutaneous transcatheter embolization for control of life-threatening pelvic hemorrhage from gestational trophoblastic disease. Obstet Gynecol. 1992; 80:571–4.
7. Takemura M, Yamasaki M, Tanaka F, Shimizu H, Okamoto E, Hisamatu K, et al. Transcatheter arterial embolization in the management of gynecological neoplasms. Gynecol Oncol. 1989; 34:38–42.

8. Moodley M, Moodley J. Transcatheter angiographic embolization for the control of massive pelvic hemorrhage due to gestational trophoblastic disease: a case series and review of the literature. Int J Gynecol Cancer. 2003; 13:94–7.

9. Keepanasseril A, Suri V, Prasad GR, Gupta V, Bagga R, Aggarwal N, et al. Management of massive hemorrhage in patients with gestational trophoblastic neoplasia by angiographic embolization: a safer alternative. J Reprod Med. 2011; 56:235–40.
10. Lim AK, Agarwal R, Seckl MJ, Newlands ES, Barrett NK, Mitchell AW. Embolization of bleeding residual uterine vascular malformations in patients with treated gestational trophoblastic tumors. Radiology. 2002; 222:640–4.

11. Berkowitz RS, Goldstein DP. Gestational trophoblastic disease. Berek JS, Novak E, editors. editors.Berek & Novak's gynecology. 14th ed.Philadelphia (PA): Lippincott Williams & Wilkins;2007. p. 1581–604.
12. Li MC. Trophoblastic disease: natural history, diagnosis, and treatment. Ann Intern Med. 1971; 74:102–12.

13. Lal A, Singhal M, Kumar S, Bag S, Singh SK, Khandelwal N. Bilateral renal and jejunal metastasis of choriocarcinoma presenting as spontaneous renal hemorrhage. Cancer Imaging. 2009; 9:56–8.

14. Lurain JR, Brewer JI, Mazur MT, Torok EE. Fatal gestational trophoblastic disease: an analysis of treatment failures. Am J Obstet Gynecol. 1982; 144:391–5.

15. Lok CA, Reekers JA, Westermann AM, Van der Velden J. Embolization for hemorrhage of liver metastases from choriocarcinoma. Gynecol Oncol. 2005; 98:506–9.

16. Menczer J, Modan M, Serr DM. Prospective follow-up of patients with hydatidiform mole. Obstet Gynecol. 1980; 55:346–9.

17. Genest DR, Laborde O, Berkowitz RS, Goldstein DP, Bernstein MR, Lage J. A clinicopathologic study of 153 cases of complete hydatidiform mole (1980–1990): histologic grade lacks prognostic significance. Obstet Gynecol. 1991; 78:402–9.
18. Flam F, Hambraeus-Jonzon K, Hansson LO, Kjaeldgaard A. Hydatidiform mole with non-metastatic pulmonary complications and a false low level of hCG. Eur J Obstet Gynecol Reprod Biol. 1998; 77:235–7.

19. Cole LA, Butler SA, Khanlian SA, Giddings A, Muller CY, Seckl MJ, et al. Gestational trophoblastic diseases: 2. Hyperglycosyl-ated hCG as a reliable marker of active neoplasia. Gynecol Oncol. 2006; 102:151–9.

20. Cole LA, Khanlian SA, Muller CY, Giddings A, Kohorn E, Berkowitz R. Gestational trophoblastic diseases: 3. Human chorionic gonadotropin-free beta-subunit, a reliable marker of placental site trophoblastic tumors. Gynecol Oncol. 2006; 102:160–4.
21. O'Reilly SM, Rustin GJ. Mismanagement of choriocarcinoma due to a false low HCG measurement. Int J Gynecol Cancer. 1993; 3:186–8.
22. Levavi H, Neri A, Bar J, Regev D, Nordenberg J, Ovadia J. “Hook effect” in complete hydatidiform molar pregnancy: a falsely low level of beta-HCG. Obstet Gynecol. 1993; 82:720–1.
23. Hoffman KL. Optimization of sandwich immunometric assay. J Clin Immunoassay. 1985; 8:237–44.
Go to : 
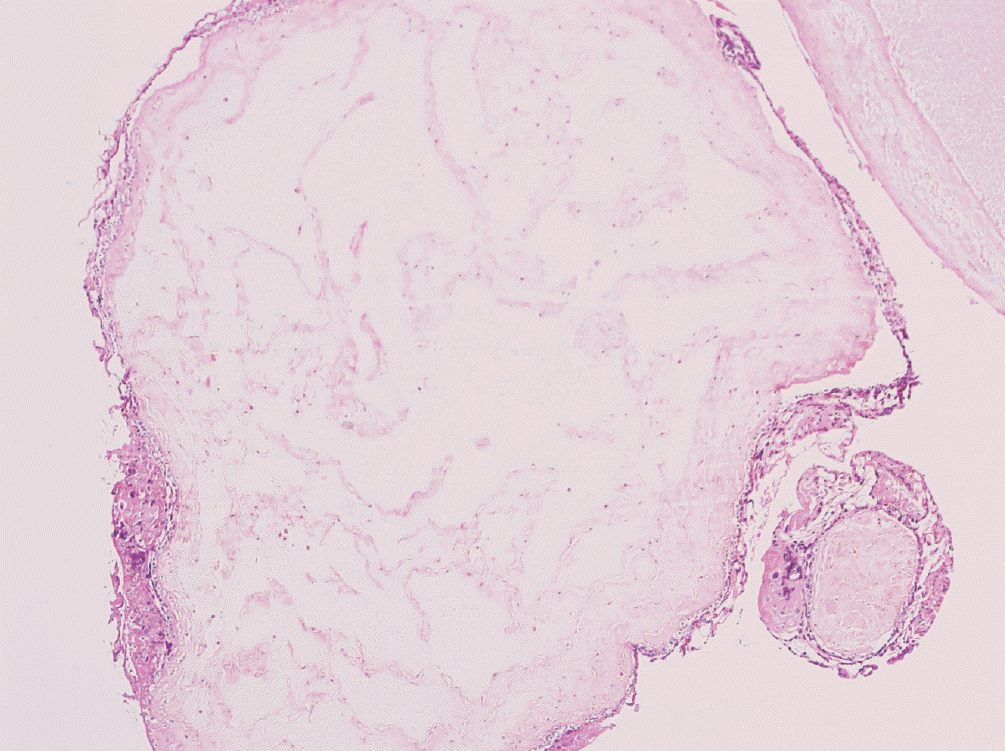 | Fig. 1.Microscopic examination of the evacuated molar tissue. Enlarged villous with central cavitation and surrounding trophoplastic hyperplasia are shown. Complete mole was diagnosed (H&E, ×100). |
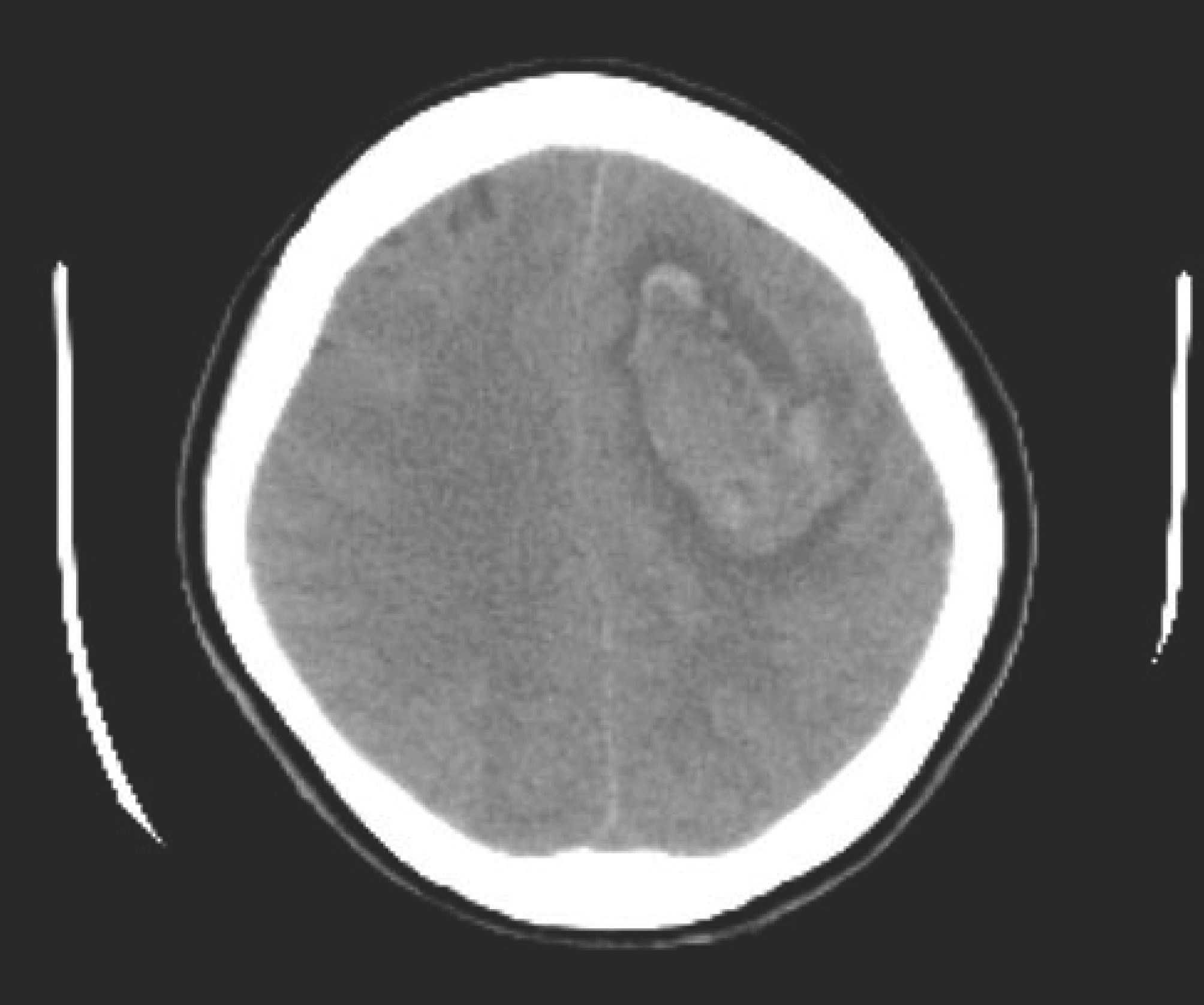
 | Fig. 2.Abdominopelvic computed tomography showing renal hemorrhage with multiple metastases. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download