Abstract
Most of superior vena cava syndrome are caused by malignant tumors involving the mediastinum (usually lung cancer or lymphoma). However, iatrogenic cause has become more important since it has become common to utilize long-term central venous catheters for chemotherapy or hyperalimentation therapy. Although the cancer patients are also at relatively high risk, there are only few case reports of superior vena cava syndrome in gynecologic cancer. We present a case of iatrogenic superior vena cava syndrome related with Hickman catheter in advanced cervical cancer patient treated with concurrent chemoradiotherapy.
Superior vena cava (SVC) syndrome was first described in 1757 in a patient with an aorta aneurysm [1]. Although in most cases, it results from direct tumor infiltration with compression, recently many other risk factors have been identified including infection and iatrogenic causes, such as pacemaker or central venous access devices. With the increasing use of long-term central venous catheters for chemotherapy in cancer patients, there have been also some case reports of catheter related SVC syndrome. There are 18 cases of SVC syndrome reported in gynecologic cancer [1-3], 7 of them are catheter-related.
Currently, concurrent chemoradiation (CCRT) is the standard therapy for locally advanced cervical cancer. Although paclitaxel and carboplatin combination therapy appears promising [4], the 5-fluorouracil (5-FU) and cisplatin (FP) combination therapy which usually requires Hickman catheter insertion is still one of the representative regimens for CCRT. [5]
We report a case of iatrogenic superior vena cava syndrome secondary to venous thrombosis produced by a Hickman catheter successfully treated with catheter removal and heparinization.
A 56-year-old woman visited the emergency room of Samsung Seoul Medical Center for massive vaginal bleeding. The patient was immediately referred to the gynecology department and underwent diagnostic endometrial and endocervical curettage. The pathology revealed as squamous cell carcinoma of uterine cervix. Although International Federation of Gynecology and Obstetrics (FIGO) stage was Ib, magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) showed a 6cm-sized endocervical mass and paraaortic lymph node metastasis. The patient was planned to receive CCRT with combination of 5-FU and cisplatin. For administration of chemotherapeutic agents and medications, a Hickman catheter was placed through the right internal jugular vein. Subsequent patency of the catheter was maintained by weekly flushing with 3 mL of heparin (10 units/mL in saline). The patient had been received CCRT with completion of four cycles of chemotherapy.
Two months after Hickman catheter insertion, the patient presented to emergency room for head trauma after loss of consciousness with extreme swelling in her face and neck. She also had complaints of mild dyspnea and cough refractory to medication. Chest X-ray (Fig. 1) and Brain CT were normal, then a chest/neck CT was performed to evaluate the obstructing tumor. Massive thrombosis was shown in SVC, right subclavian vein, left brachiocephalic vein and azygous vein (Fig. 2). A diagnosis of superior vena cava syndrome was made. Vascular surgical part and radiological intervention consultation were obtained and thrombolytic therapy was instituted. The catheter was removed, and the patient was treated with systemic heparinization for 5 days until the international normalized ratio (INR) reaches therapeutic level.
Anticoagulation was also begun with warfarin. She had symptomatic relief and obvious improvement of facial edema within 3 days after beginning of heparinization. Follow-up CT angiography showed remaining thrombus, and the patient continued on oral anticoagulation for additional 3 months.
Superior vena cava syndrome has been rarely described in patients with gynecologic malignancies. There has been a few case reports only [2] and no previous description about SVC syndrome in gynecologic malignancy in Korea. This is the first case of SVC syndrome in gynecologic cancer in Korea.
The most common underlying cause of SVC syndrome in adults is malignancy involving the mediastinum, constituting greater than 90% of cases. Only a small proportion of the cases of SVC syndrome were caused by benign etiology (Table 1) [6]. However, recently iatrogenic cause in case of central venous access devices has become important [7,8].
The initial diagnosis of superior vena cava syndrome is clinical without extensive laboratory testing [1,3]. This clinical diagnosis is confirmed by imaging study, usually chest CT scan which also helps to evaluate the underlying disease and severity of thrombosis. Common symptoms include facial swelling, dyspnea, cough, arm swelling, and orthopnea (Table 2) [9]. This case describes a patient with both signs (venous distension and facial/arm swelling) and symptoms (dyspnea, cough, loss of consciousness) commonly associated with superior vena cava syndrome.
Treatment of SVC syndrome primarily depends on the underlying cause. In cases of SVC obstruction secondary to thrombosis, thrombolytic therapy or anticoagulant therapy should be instituted [1]. Thrombolytic therapy does offer the most rapid and complete method [3]. When a venous catheter has been implicated as a cause of thrombosis, catheter removal has been reported. However, thrombolytic therapy following stent dilation with central catheter left in situ has been reported recently [1]. In case of tumor obstruction, tumor resection, irradiation, stents, and chemotherapy have been utilized successfully in the short term. Especially, patients with recurrent SVC syndrome can be treated with repeated percutaneous intervention [9].
The presence of a thrombosis in the superior vena cava associated with a venous catheter does not exclude the presence of active tumor in the mediastinum. Therefore, evaluation of the mediastinum as well as superior vena cava is mandatory in the work-up of patients with superior vena cava syndrome [7]. In our case, there is no evidence of mediastinal tumor although other reported cases had mediastinal metastasis.
Anticoagulation may be important because cancer patient are in a hypercoagulable state. However, the risk of thrombosis has to be balanced against possible problems arising from the combination of anticoagulation and thrombocytopenia resulting from chemotherapy [10,11]. According to the large scale of Cochrane review about prophylactic anticoagulation for cancer patients with central venous catheter, there are no statistically significant effect of heparin or vitamin K antagonists [12-14]. Otherwise, Minassian et al. reported that subcutaneous port had much lower rates of complications including thrombotic occlusion, infection, and catheter failure [15].
We report a case of SVC syndrome related to Hickman catheter in the advanced cervical cancer patient treated with concurrent chemoradiotherapy. Hickman catheter is important for gynecologic malignancy cancer patients for administration of chemotherapeutic agent or total parenteral nutrition. Central venous catheter including Hickman catheter leads these patients to more hypercoagulable state [10]. However, there are no evidence that thromboprophylaxis for patients with central venous catheter is beneficial.
In conclusion, physicians must assume a SVC syndrome when treating cancer patients with central venous catheter present facial swelling, dyspnea, cough or orthopnea. The choice of treatment depends on patients' symptoms or causes. Prophylactic anticoagulants is controversial at present, however thrombolysis or stent insertion can be an effective treatment option.
Figures and Tables
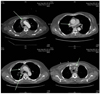 | Fig. 2Chest computed tomography revealed thrombosis in superior vena cava, right subclavian vein, left brachiocephalic vein and azygous vein without evidence of cancer metastasis. |
Table 1
Historical etiology of superior vena cava syndrome (form Schifferdecker et al. with permission from Wiley-Liss, Inc. [6])

References
1. Griffin D, Martino MA, Hoffman MS. Superior vena cava syndrome during chemotherapy for stage 3c fallopian tube adenocarcinoma. Gynecol Oncol. 2004. 93:257–259.
2. Matthews JA, Blake HA, Hall DJ. Iatrogenic superior vena cava syndrome treated with streptokinase. Gynecol Oncol. 1987. 26:119–122.
3. Puleo JG, Clarke-Pearson DL, Smith EB, Barnard DE, Creasman WT. Superior vena cava syndrome associated with gynecologic malignancy. Gynecol Oncol. 1986. 23:59–64.
4. Kitagawa R, Katsumata N, Ando M, Shimizu C, Fujiwara Y, Yoshikawa H, et al. A multi-institutional phase II trial of paclitaxel and carboplatin in the treatment of advanced or recurrent cervical cancer. Gynecologic Oncology. 2012. 125:307–311.
5. Choi IJ, Cha MS, Park ES, Han MS, Choi Y, Je GH, et al. The efficacy of concurrent cisplatin and 5-flurouracil chemotherapy and radiation therapy for locally advanced cancer of the uterine cervix. J Gynecol Oncol. 2008. 19:129–134.
6. Schifferdecker B, Shaw JA, Piemonte TC, Eisenhauer AC. Nonmalignant superior vena cava syndrome: pathophysiology and management. Catheter Cardiovasc Interv. 2005. 65:416–423.
7. Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer. 1984. 54:376–378.
8. Estes JM, Rocconi R, Straughn JM, Bhoola S, Leath CA, Alvarez RD, et al. Complications of indwelling venous access devices in patients with gynecologic malignancies. Gynecol Oncol. 2003. 91:591–595.
9. Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med. 1999. 66:59–61.
10. Preston CI, Poynton CH, Williams LB. Intermittent superior vena cava syndrome caused by a Hickman catheter. Clin Oncol (R Coll Radiol). 1992. 4:60–61.
11. Roberts JR, Bueno R, Sugarbaker DJ. Multimodality treatment of malignant superior vena caval syndrome. Chest. 1999. 116:835–837.
12. Akl EA, Vasireddi SR, Gunukula S, Yosuico VE, Barba M, Sperati F, et al. Anticoagulation for patients with cancer and central venous catheters. Cochrane Database Syst Rev. 2011. (2):CD006468.
13. Young AM, Billingham LJ, Begum G, Kerr DJ, Hughes AI, Rea DW, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009. 373:567–574.
14. Prandoni P. Prophylaxis of catheter-related thrombosis in cancer patients. Lancet. 2009. 373:523–524.
15. Minassian VA, Sood AK, Lowe P, Sorosky JI, Al-Jurf AS, Buller RE. Longterm central venous access in gynecologic cancer patients. J Am Coll Surg. 2000. 191:403–409.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download