Abstract
A 28-year-old female presented with Rathke's cleft cysts (RCC) manifesting as primary amenorrhea and no development of secondary sexual characteristics. She had no symptoms of headache, visual disturbance, anosmia or galactorrhea.. The endocrinologic study demonstrated partial hypopituitarism. Magnetic resonance imaging revealed about 1.5 cm sized sellar and suprasellar cystic tumor, extending into the pituitary stalk. Its tentative diagnosis was benign pituitary cystic tumor, such as RCC. She underwent surgery by a transsphenoidal approach. Histological examination revealed a ciliated columnar epithelium those consistent with RCC. RCC is rarely symptomatic, but mechanical compression by the cyst or inflammation itself causes headache, visual field defects, or symptoms of pituitary dysfunction. The present case shows that RCC may manifest as hypogonadotropic hypogonadism.
Rathke's cleft cysts (RCC) are intrasellar or suprasellar cysts derived from the remnants of Rathke's pouch in the intermediate lobe region of the pituitary gland [1,2]. They are lined by a single layer of ciliated cuboidal or columnar epithelium including cilia and goblet cells, which secrete mucus into the cyst [3]. RCC are relatively common and have been found in up to 13-22% in autopsies [4]. Although RCC are usually asymptomatic, they sometimes grow large enough to compress the surrounding structure and become symptomatic [5]. RCC manifest as headache, visual impairment or pituitary dysfunction [2,3]. We report a case of RCC manifesting as primary amenorrhea.
In November 2008, a 28-year-old female visited the gynecology clinic of CHA Bundang Medical Center for the evaluation of primary amenorrhea. Four years before this visit, she had presented other university hospital, complaining of primary amenorrhea and no development of breast and pubic hair. At that time the endocrine studies had represented low levels of estradiol (10 ng/mL), luteinizing hormone (LH, 1.5 mIU/mL) and follicle stimulating hormone (FSH, 2.4 mIU/mL), indicating hypogonadotropic hypogonadism. Based on this result, she had undergone the hormone replacement therapy (estradiol valerate 2 mg and cyproterone 1 mg) and came to have withdrawal bleeding and the development of breast and pubic hair.
When she visited our clinic, she had no symptoms of headache, visual disturbance, anosmia or galactorrhea. The main findings on examination were as follows: height 152 cm, weight 42 kg. Her blood pressure was 100/60 mm Hg and pulse rate was 76/min. The breast development was Tanner stage 4 and the pubic hair development was Tanner stage 2. The external genitalia looked estrogenized. Gynecological sonography showed atrophied uterus and small right ovary (uterus, 2.0×5.3 cm; right ovary, 1.6×1.2 cm). The left ovary was not seen. Blood chemistry showed no abnormal findings. Laboratory tests showed normal thyroid function (thyroid stimulating hormone [TSH] 3.39 uIU/mL, free T4 1.08 ng/dL). Anterior pituitary function was investigated with a set of two tests with insulin and luteinizing hormone releasing hormone (LHRH) (Table 1). Although insulin-induced hypoglycemia increased serum cortisol level, it failed to increase growth hormone (GH) level. FSH and LH responses to LHRH were impaired [6,7].
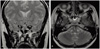
Magnetic resonance imaging (MRI) revealed about 1.5 cm sized sellar and suprasellar cystic tumor, extending into stalk without any solid portion (Fig. 1). The normal pituitary gland was compressed peripherally and small in volume. The normal T1 high signal in posterior lobe was not definite. Its tentative diagnosis was benign pituitary cystic tumor, such as RCC.
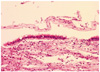
In July 2009, she was admitted to the department of neurosurgery of CHA Bundang Medical Center for the operation. On admission, the neurologic examination was done but particular pathologic findings were not observed. The navigator assisted surgery was performed via the transsphenoidal approach. Yellowish brown colored, fragile tumor was removed out with suction and curettage. The wall of the tumor was excised. Histological examination confirmed RCC, a ciliated columnar epithelium lined cyst (Fig. 2). The postoperative MRI revealed sellar portion of RCC was removed, but suprasellar portion was remained.
She was discharged without postoperative complications and subsequently treated as an out-patient. She didn't get menses in five months after the operation yet. Therefore, she restarted to take the hormone replacement therapy and calcium + vitamin-D replacement. The endocrinologic study demonstrated partial hypopituitarism. Anterior pituitary function was investigated postoperatively (Table 2). FSH and LH responses to LHRH were still impaired and TSH response to thyrotropin releasing hormone was impaired too. The thyroid function test showed secondary hypothyroidism (TSH 0.03 uIU/mL, free T4 0.81 ng/dL). Therefore, she was given synthroid 0.05 mg daily. And she plans to take the ovulation induction in the infertility department of CHA Bundang Medical Center soon.
RCC shows few symptoms with the size of less than 1 cm. However, when it grows more than 1 cm or inflammatory changes develop, various symptoms may occur [8]. Mechanical compression of the pituitary gland by the cyst, as well as inflammation itself, may play a major role in causing pituitary dysfunction in RCC patients [8]. The persisting inflammation reaction to ruptured RCC fluid contents induces significant interstitial fibrosis and atrophy of pituitary gland. The reserve of the pituitary gland decreases, resulting in an empty sella filled with necrotic tissue in the final stages [3,4]. In small number of studies, the hypopituitarism was found in 39-100% of patients with RCC [8-10].
The prevalent endocrine symptoms in RCC patients were impotence or diminished libido (67%), amenorrhea or oligomenorrhea (48%), hyperprolactinemia (46%), galactorrhea (35%), and reduced secondary sex features (23%) [9]. Yoshida et al. [11] reported that the mean age of the patients was 38 years old, and the highest frequency was in the fifth decade with marked female preponderance. Endocrinological presentation of RCC, such as amenorrhea, is thought to be the major cause of marked female preponderance [6,8]. Mukherjee et al. [1] presented the details of 12 patients with RCC. One of them presented with headache, galactorrhea, and oligomenorrhea. Serum prolactin was elevated. Hyperprolactinemia is considered to be induced as a result of a compressed or infiltrated pituitary stalk. Hong et al. [12] reported a case about 43-year-old woman with visual disturbance and oligomenorrhea. Roncaroli et al. [13] presented a 37-year-old woman with a 3-year-history of headache and amenorrhea caused by ruptured RCC. She had experienced normal sexual maturation, and her medical history was unremarkable.
Surgical treatment is generally recommended for the symptomatic RCC. Simple drainage and partial excision of the cyst wall is the treatment of choice [6,7]. Generally, the prognosis after surgery seems to be good even though the cyst recurs in some cases [6]. Most (64-100%) of RCC patients had improvement of amenorrhea, galactorrhea, and oligomenorrhea after surgical treatment [8,9]. Therefore, the surgical treatment is recommended even when the patient has only mild symptoms or signs, including pituitary dysfunction [8]. In this case, however, the patient didn't get menses in five months after the operation yet. FSH and LH responses to LHRH were persistently impaired postoperatively. Of note, the postoperative MRI revealed the remnant of RCC in suprasellar area. This portion of RCC might be the cause of her persistent amenorrhea and hypogonadotropic hypogonadism. Nevertheless, it is also possible that the mechanical compression by the cyst or inflammation caused irreversible functional change of pituitary gland before surgical treatment. The postoperative thyroid function test showed secondary hypothyroidism though the preoperative one was normal. The progressive change after the initial evaluation and or the surgical damage may cause secondary hypothyroidism though it was normal at initial test. This is a very rare case of RCC causing primary amenorrhea and delayed puberty.
Figures and Tables
Fig. 1
Coronal (A) and transverse (B) T2-weighted magnetic resonance imaging demonstrating sellar and suprasellar cystic tumor extending into stalk.
References
1. Mukherjee JJ, Islam N, Kaltsas G, Lowe DG, Charlesworth M, Afshar F, et al. Clinical, radiological and pathological features of patients with Rathke's cleft cysts: tumors that may recur. J Clin Endocrinol Metab. 1997. 82:2357–2362.
2. Miyajima Y, Oka H, Utsuki S, Kondo K, Sato K, Fujii K. Symptomatic Rathke's cleft cyst with cavernous sinus syndrome. Neurol Med Chir (Tokyo). 2007. 47:576–578.
3. Ogawa Y, Tominaga T, Ikeda H. Clinicopathological and endocrinological study of Rathke's cleft cyst manifesting as hyponatremia. Neurol Med Chir (Tokyo). 2007. 47:58–63.
4. Schittenhelm J, Beschorner R, Psaras T, Capper D, Nägele T, Meyermann R, et al. Rathke's cleft cyst rupture as potential initial event of a secondary perifocal lymphocytic hypophysitis: proposal of an unusual pathogenetic event and review of the literature. Neurosurg Rev. 2008. 31:157–163.
5. Tanaka T, Oka H, Kawano N, Kobayashi I, Saegusa H, Fujii K. Juvenile symptomatic Rathke's cleft cyst--case report. Neurol Med Chir (Tokyo). 1998. 38:578–581.
6. Elias AN, Valenta LJ. A combined anterior pituitary stimulation test: experience with 285 individuals. J Natl Med Assoc. 1987. 79:1185–1197.
7. Mortimer CH, Yeo T. Gonadotrophin-releasing hormone. J Clin Pathol Suppl (Assoc Clin Pathol). 1976. 7:46–54.
8. Eguchi K, Uozumi T, Arita K, Kurisu K, Yano T, Sumida M, et al. Pituitary function in patients with Rathke's cleft cyst: significance of surgical management. Endocr J. 1994. 41:535–540.
9. Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke's cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999. 84:3972–3982.
10. Voelker JL, Campbell RL, Muller J. Clinical, radiographic, and pathological features of symptomatic Rathke's cleft cysts. J Neurosurg. 1991. 74:535–544.
11. Yoshida J, Kobayashi T, Kageyama N, Kanzaki M. Symptomatic Rathke's cleft cyst. Morphological study with light and electron microscopy and tissue culture. J Neurosurg. 1977. 47:451–458.
12. Hong SH, Gwak HS, Jung HW. Recurrent sympomatic Rathke's cleft cyst: case report and review of the literature. J Korean Neurosurg Soc. 2000. 29:286–290.
13. Roncaroli F, Bacci A, Frank G, Calbucci F. Granulomatous hypophysitis caused by a ruptured intrasellar Rathke's cleft cyst: report of a case and review of the literature. Neurosurgery. 1998. 43:146–149.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download