Abstract
Yolk sac tumor (YST) is one of the rare malignant germ cell tumor and usually occurs in gonad. Extragondal sites of YSTs are reported in mediastinum, vagina, brain, and retroperitoneum but are extremely rarely in omentum. The clinicopathologic feature of primary omental YST is not well known and there are only 5 cases reported currently. Recently we experienced a primary YST of omentum in 27-year-old woman who was performed exploratory laparotomy due to abdominal distension and pain. She has remained free of diseases for 2 years with normal menstruation after the fertility-saving surgery and postoperative adjuvant chemotherapy with bleomycin, etoposide, cisplatin regimen. The subject of primary YST of omentum is reviewed, and the possible histogenesis of the tumor is discussed.
Among the malignant germ cell tumor, yolk sac tumor (YST) is known to be the third most common malignant germ cell tumor, following the dysgerminoma and immature teratoma [1-3]. YST simulate the yolk sac histologically and functionally in production of alphafetoprotein (AFP) [4]. Although most of YST occurs in the ovaries, 10 to 15% of YST may arise in extragonadal sites, such as the mediastinum, the pineal region, and sacrococcygeal region and the female reproductive tract [5]. Among them primary YST of omentum is extremely rare and only 5 cases are reported currently, and the pathogenesis and the clinical characteristics are unknown [1,2,4-6].
We report a case of primary YST of the omentum in 27-year-old women and discuss the clinical and histologic features in conjunction with a review of the literature.
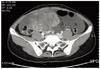
A 27-year-old woman, single of nullipara, was referred to department of gynecology due to aggravating abdomen distension and pain that develop incidentally a month ago. On history taking, her mentral cycle was regular with 28 day cycle without any menorrhagia or dysmenorrhea. On physical examination, fetal head sized pelvic mass was palpated with a tenderness but without rebound tenderness. Laboratory test showed negative urine-human chorionic gonado trophin and no leukocytosis. Computerized tomography (CT) scan showed a large lobulating contoured pelvic mass infiltrating the omentum with ascites and a low attenuating nodule at right pericardiophrenic area (Fig. 1).
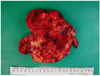
On the exploratory laparotomy, there was a 13 × 12 cm multiloculated solid, yellowish-grey mass in the greater omentum with multiple small nodules measuring 1-1.5 cm in greatest dimension. Another 7 × 5 cm white yellowish and friable multiloculated mass was found in cul-de-sac with small amount of ascites. The uterus, both fallopian tube and right ovary are grossly normal, but there was a 1.5 × 0.5 cm small nodule at left ovary. The frozen biopsy showed a malignancy favoring YST. In abdominal and pelvic cavity, disseminating bean sized small nodules were also found.
Debulking surgery with the resection of left ovarian surface masses and the cul-de-sac mass, pelvic lymphadenectomy, omentectomy and resection and reanastomosis of the small bowel were performed saving the uterus and both ovary. Emergency laboratory test showed an elevated alpha fetoprotein (AFP) 6,065 U/mL.
On pathological examination, the omental mass measured 13.5 × 13 × 7 cm and 603 g weight. The cut surface is white, soft, hemorrhagic, and partly shows necrosis (Fig. 2).
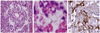
Histological evaluation showed the YST exhibiting the Schiller-Duval body (Fig. 3A) and hyaline globules (Fig. 3B). Special immunohistochemical staining of the tumor showed positive for AFP (Fig. 3C), cytokeratin and negative for β-HCG.
On the 10th day after surgery, adjuvant bleomycin, etoposide, cisplatin (BEP) chemotherapy consisting of bleomycin (20 mg iv on days 2, 9, and 16), etoposide (100 mg/m2 iv for 5 consecutive days), and cisplatin (15 mg/m2 iv for 5 consecutive days) was begun. A total of four cycles of chemotherapy were repeated every 3 weeks. The patient showed complete response after 6 cycles of chemotherapy and showed no evidence of disease on physical examination, serum AFP level, and abdomino-pelvic CT scan for 2 years.
The extragonadal YST is rare malignant germ cell tumor arising outside ovary or testes. The most common site of extragonadal YST are reported in sacrococcygeal region followed by anterior mediastinum, vagina, vulva, cervix, and uterine corpus [4].
However, primary YST of omentum is extremely rare and only 5 cases are reported currently [1,2,4-6].
Omental YST shows histologic characteristics similar to that of gonadal YST such as microcystic, endodermal sinus, solid, alveolarglandular, polivesicular vitelline, myxomatous, papillary, macrocystic, hepatoid, and glandular or primitive endoderm patterns [5]. Schiff positive intracytoplasmic and extracytoplasmic globules are typical features of YST and these globules are usually positive immunohistochemically for AFP, A1AT, transferrin, and basement membrane components such as fibronectin, type IV collagen, vimentin, and laminin [4,5]. In our case, typical histologic characteristics of YST such as Schiller-Duval bodies and positive immunohistochemical staining for AFP and cytokeratin.
The histogenesis of extragonadal YST remains controversial. One hypothesis is that these tumors arise from aberrant differentiation of somatic cells, and the other one is that the germ cells have been arrested in their embryonic migration. The arrested embryonic migration hypothesis explains that when the primitive gonadal ridge expanded to the region of the external genitals, some of germ cell arrested anywhere along the migration course and can be the possible site of germ cell tumor in the future [4]. Metastasis from an occult focus in the ovary may contribute to primary YST of omentum, but in our case, thorough pathologic examination has excluded this possibility.
In this case, omental mass was huge main mass and left ovarian mass was located at ovarian surface without invasion, grossly. Although in pathologic examination small nodule at left ovary shows yolk sac tumor, ovarian capsule infiltration was not confirmed. Mass of ovary was one of metastatic seeding nodules as like those disseminated in abdominal and pelvic cavity.
Generally, gonadal YST is a disease with a peak incidence at 20 years [5], however, the ages of in w primary YST of omentum omen previous reported were is 37, 44, 45, and 46 years implicating a rather older women's disease [2,4,6]. However, primary YST of omentum in our report showed a general young age of gonadal YSTs.
Because omental YST also affects women of child bearing age, the treatment of ometnal YST may not different from that of gondal YST [7]. Any gross metastases should be resected if possible, and "fertility preserving" procedure can be considered even when the tumor may remain after surgery [3,8]. Preservation of uterus and ovaries are strongly recommended because the high response rate of chemotherapy with BEP [3,9,10]. In previous report, the average age of the 4 women with a primary YST of omentum was 43 years and there was no need to 'fertility-saving surgery'. Actually total hysterectomy was performed in all the 4 women with primary YST of omentum [2,4,6]. However, in this report, we performed fertility saving surgery consisting of removal of the pelvic tumor, pelvic lymphadenectomy, omentectomy and resection of abdominal metastases with preservation of the uterus and both ovaries. Although the child bearing outcome is not confirmed yet, this is the first report that fertility saving surgery was performed in primary YST of omentum.
Primary YST of omentum responds well to combination chemotherapy [2,4,6]. A regimen of BEP has been a major advance in the therapy of advanced and localized germ cell tumors [3]. Our patient also showed complete response with 6 cycyles of BEP combination chemotherapy.
Although long term outcome of omental YST is not well known, all the 5 patients with primary YST of omentum reported showed a favorable outcome [2,4,6]. In this report, the patient showed no evidence of disease on physical examination and serum a-fetoprotein level and imaging studies for 2 years without any menstrual abnormality.
In conclusion, we report a 27-year-old woman with a primary YST of omentum successfully with fertility saving surgery and postoperative adjuvant chemotherapy with a brief review of literature.
Figures and Tables
Fig. 1
Computerised tonography showed a large lobulating abdominal mass infiltrating the omentum with small amount of ascites.
References
1. Rossi R, Stacchiotti D, Bernardini MG, Calvieri G, Lo Voi R. Primary yolk sac tumor of the endometrium: a case report and review of the literature. Am J Obstet Gynecol. 2011. 204:e3–e4.
2. Park NH, Ryu SY, Park IA, Kang SB, Lee HP. Primary endodermal sinus tumor of the omentum. Gynecol Oncol. 1999. 72:427–430.
3. Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev. 2008. 34:427–441.
4. Kim SW, Park JH, Lim MC, Park JY, Yoo CW, Park SY. Primary yolk sac tumor of the omentum: a case report and review of the literature. Arch Gynecol Obstet. 2009. 279:189–192.
5. Pasternack T, Shaco-Levy R, Wiznitzer A, Piura B. Extraovarian pelvic yolk sac tumor: case report and review of published work. J Obstet Gynaecol Res. 2008. 34:739–744.
6. Zhang B, Gao S, Chen Y, Wu Y. Primary yolk sac tumor arising in the pancreas with hepatic metastasis: a case report. Korean J Radiol. 2010. 11:472–475.
7. Piura B, Dgani R, Zalel Y, Nemet D, Yanai-Inbar I, Cohen Y, et al. Malignant germ cell tumors of the ovary: a study of 20 cases. J Surg Oncol. 1995. 59:155–161.
8. Berek JS, Hacker NF. Berek and Hacker's gynecologic oncology. 2010. Philadelphia (PA): Lippincott Williams & Wilkins.
9. Weinberg LE, Lurain JR, Singh DK, Schink JC. Survival and reproductive outcomes in women treated for malignant ovarian germ cell tumors. Gynecol Oncol. 2011. 121:285–289.
10. Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005. 17:299–308.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download