Abstract
Large cell neuroendocrine carcinoma (LCNEC) of the ovary is a rare entity and is frequently associated with ovarian surface epithelial tumors. Since Collins et al. described the first case of mixed neuroendocrine and mucinous carcinoma, a few more cases of primary ovarian LCNECs have been reported. The prognosis of LCNECs is generally very poor even when the diagnosis is made at an early stage. A 40-year-old woman presented with a pelvic mass measuring 30 cm in diameter. She underwent an exploratory laparotomy for resection of the pelvic mass and staging if needed. After the operation, she was diagnosed of stage Ia LCNEC associated with mucinous tumor (including benign, borderline, malignant tumor) of left ovary. The patient received six cycles of paclitaxel-carboplatin chemotherapy postoperatively. There have been no signs of local tumor recurrence or metastasis at follow-up examinations during the first 8 months after the operation.
Neuroendocrine differentiation may be expressed in a variety of ovarian tumors including surface epithelial tumor, Sertoli-Leydig cell tumors, teratomas, carcinoid tumor, small cell carcinoma of pulmonary type, small cell carcinoma of hypercalcemic type, and non-small cell undifferentiated carcinoma of neuroendocrine type [1]. According to the definition of World Health Organization, primary ovarian large cell neuroendocrine carcinoma (LCNEC) is synonymous with 'undifferentiated carcinoma of non-small cell neuroendocrine type' [2]. Its prognosis is generally very poor even when the diagnosis is made at an early stage. Most patients have died of disseminated disease within one year after primary operation even extensive chemotherapy. And the longest recorded survival is 68 months [3]. Due to the rarity of LCNECs, a general consensus for optimal treatment has yet to emerge. We report a primary ovarian large cell neuroendocrine carcinoma associated with ovarian epithelial micinous tumor (including benign, borderling, malignant tumor).
A 40-year-old multiparous woman was referred for abdominal distension sustained for 3 months. Physical examination revealed a firm, round, regular mass in the abdomen. A computed tomography (CT) scan of her abdomen and pelvis showed about 30 cm sized multilocular cystic tumor with enhancing portions in left ovary (Fig. 1). There was no retroperitoneal lymphadenopathy or peritoneal deposits and no evidence of metastatic disease on the CT scan.
At the time of admission, her vital signs were all within normal range and her general condition was good. Her hemoglobin level, red cell volume, leukocyte and platelet count were all normal level. Other urinalysis and blood chemistry results were within normal rages. Serum level of the tumor markers CA-125 and CA19-9 were 88.95 U/mL and 3.49 U/mL, the CA-125 was slightly increased.
The patient underwent an exploratory laparotomy and was found to have a 30 cm diametered multilocular cystic and solid left ovarian mass. Frozen section was done during operation showed 'adenocarcinoma'. So total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy and appendectomy were done along with both pelvic and para-aortic lymph node dissection. There was no gross visible lesion on any other abdominal or pelvic organs.
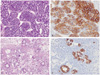
Pathologic examination confirmed a LCNEC associated with mucinous tumor component (incluing benign, borderline, and malignant tumor). The tumor had two discernable morphologic features: neuroendocrine carcinoma composing approximately 10% of the tumor mass (Fig 2A), and the remaining being ovarian mucinous carcinoma type associated with benign and borderline mucinous tumor (Fig 2C). The LCNEC cells were positive for synaptophysin, chromogranin, CD56 (Fig 2B). The ovarian epithelial component of the tumor was positive cytokeratin 7 and CA-125 (Fig 2D). The uterus, right ovary, appendix and lymph nodes were all free of tumor, so the tumor was classified as International Federation of Gynecology stage Ia.
She have been received six courses of chemotherapy. After chemotherapy, she got a positron emission tomography-computed tomography to evaluate the response to chemotherapy. After 8 months a follow-up, there was no evidence of metastatic disease.
Neuroendocrine tumors are neoplasms with a broad range of morphologic patterns, grade of differentiation and biologic behavior that share common features of neuroendocrine programming. Neuroendocrine tumors contain various amount of molecules involved in regulated release of neuropeptide, neurotransmitters and hormones. Most neuroendocrine tumors are of endodermal derivation and rare entities of rare entities of true neural crest origin. They may occur in every topographic location, with a predilection of lung, intestine and pancreas. Other primary organs include the skin, salivary gland, prostate and various sites in the urinary, genital and biliary tracts [4].
Ovarian LCNECs are very rare tumor [3]. Neuroendocrine cells have been identified in normal epithelium of the female genital tract. Ovarian neuroendocrine tumors may develop from non-neuroendocrine cells in which there has been activation of genes that promote neuroendocrine differentiation [5].
On the diagnosis of the LCNEC, some other tumors as primary or metastatic carcinoid tumor, small cell carcinomas of pulmonary type, small cell carcinoma of hypercalcemic type and some kinds of specific ovarian tumors should be differentiated. Primary or metastatic carcinoid tumors can be differentiated from LCNECs by their lower mitotic activity, cytological uniformity, organoid architectural patterns, and absence of necrosis. Small cell carcinomas of pulmonary type are distinguished by their smaller cell size and less-intense immunohistochemical reactivity for cytokeratin and chromogranin. Also small cell carcinomas of hypercalcemic type are distinguished by their large neoplastic cells. In addition, the large neoplastic cells in these tumors often display pale intracytoplasmic hyaline globules, which is not a feature of LCNECs [6].
Specifically, teratomas, Sertoli-Leydig cell tumors may all show neuroendocrine differentiation. These biphasic tumors can be distinguished from LCNEC by recognising the non-neuroendocrine component [7].
The neuroendocrine differentiation of the tumors can be confirmed by immunohisochemical stainings that is positive for chromogranin, synaptophysin or other neuroendocrine markers [8].
The LCNEC component in this case had morphological characteristics typical of neuroendocrine differentiation and an immunohistochemical profile that was positive for neuroendocrine differentiation and negative for epithelial markers. The ovarian epithelial tumor consisted of mucinous adenocarcinoma, borderline and benign mucinous tumor.
Given the rarity of the condition, there are limited data to guide treatment of LCNECs of ovary. Most published reports contain a few patients, and there are no prospective trials. Thirty cases have been reported so far in the literature. Almost patients were taken primary surgery, that was required for definitive tissue diagnosis, staging and tumor debulking. Follow-up was available in 26 of the 31 reported cases (incluing this case). Twenty five patients were taken chemotherapy.
The optimal chemotherapy regimen for LCNECs has not been established. Regimens used for LCNECs are cisplatinum-based chemotherapy or paclitaxel-carboplatin based chemotherapy [9,10]. Patients usually follow an extremely lethal outcome. Most patients (18 of the 26) died of disease within 1 year of diagnosis despite extensive surgery and adjuvant chemotherapy [3]. Only one patient was taken radiation therapy, so the effect of radiation therapy has not proven. Also due to the rarity of LCNECs, the effect of chemotherapy on long term survival has not yet to emerge. To establish optimal therapeutic guidelines on these tumors, additional cases have to be collected and analyzed inter-institutionally. In summary, LCNEC of the ovary is rare, under-recognised and is associated with a poor prognosis. Our patient received postoperative paclitaxel and carboplatin chemotherapy and is alive without any evidence of disease for 8 months after operation.
Figures and Tables
References
1. Eichhorn JH, Young RH. Neuroendocrine tumors of the genital tract. Am J Clin Pathol. 2001. 115:Suppl. S94–S112.
2. Eichhorn JH, Young RH, Scully RE. Primary ovarian small cell carcinoma of pulmonary type. A clinicopathologic, immunohistologic, and flow cytometric analysis of 11 cases. Am J Surg Pathol. 1992. 16:926–938.
3. Draganova-Tacheva RA, Khurana JS, Huang Y, Hernandez E, Zhang X. Large cell neuroendocrine carcinoma of the ovary associated with serous carcinoma with mucin production: a case report and literature review. Int J Clin Exp Pathol. 2009. 2:304–309.
4. Schwab M. Encyclopedic reference of cancer. 2001. New York (NY): Springer.
5. Collins RJ, Cheung A, Ngan HY, Wong LC, Chan SY, Ma HK. Primary mixed neuroendocrine and mucinous carcinoma of the ovary. Arch Gynecol Obstet. 1991. 248:139–143.
6. Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol. 1994. 18:1102–1116.
7. Veras E, Deavers MT, Silva EG, Malpica A. Ovarian nonsmall cell neuroendocrine carcinoma: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol. 2007. 31:774–782.
8. Roth LM, Tsubara A, Dietel M, Senzaki H. Tavassoli FA, Devilee P, editors. Miscellaneous tumors and tumor-like conditions of the ovary. Patholoty and genetics of tumors of the breast and female genital organs. World Health Organization Classification of Tumors. 2003. Lyon: IARC Press;182–190.
9. Tsuji T, Togami S, Shintomo N, Fukamachi N, Douchi T, Taguchi S. Ovarian large cell neuroendocrine carcinoma. J Obstet Gynaecol Res. 2008. 34:726–730.
10. Lindboe CF. Large cell neuroendocrine carcinoma of the ovary. APMIS. 2007. 115:169–176.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download