Abstract
Primary retroperitoneal mucinous cystadenocarcinoma is an extremely rare tumor. Preoperative diagnosis is very difficult and the treatment remains controversial. A 37-year-old Korean woman (gravida 0) presented with a huge abdominal mass. Computed tomography scan revealed an 18 × 11 cm sized unilocular cyst with irregular wall thickening and solid component at right adnexa. Serum CA 19-9 was slightly elevated (37.05 U/mL). At laparotomy, a huge right retroperitoneal cystic tumor originating from right paracolic gutter was found. Frozen section of the cystic tumor revealed a mucinous cystadenocarcinoma. Because the patient wished to remain fertile, fertility sparing surgery was performed. Microscopically, no evidence of metastasis was found and no further treatment was given. Six months after surgery, she has no evidence of recurrence. Fertility-sparing surgery should be considered for women with primary retroperitoneal mucinous cystadenocarcinoma, who wish to remain fertile.
Primary retroperitoneal mucinous cystadenocarcinoma is an extremely rare tumor. To date, about 50 cases have been reported [1]. The histogenesis of this unusual neoplasm is not clear. In the majority of cases, preoperative diagnosis is not possible because radiologic study such as ultrasonography or computed tomography (CT) scan is not able to distinguish the exact origin of the lesion. Due to its rarity, the treatment of primary retroperitoneal mucinous cystadenocarcinoma remains controversial. Most of these cases were treated with radical surgery, including total hysterectomy and bilateral salpingo-oophorectomy with enucleation of retroperitoneal tumor [2]. But, fertility-sparing surgery also have been adopted for the treatment of primary retroperitoneal mucinous cystadenocarcinoma and showed good prognosis [1,2]. Here, we report on a case of primary retroperitoneal mucinous cystadenocarcinoma treated with fertility-sparing surgery.
A 37-year-old Korean woman (gravida 0) presented with a huge abdominal mass. The patient reported that she had had abdominal discomfort since 1 month ago and visited local clinic. On abdominal ultrasonography, a huge abdominal mass was detected and she was referred to department of gynecology. Her previous menstrual history was regular and past medical history was noncontributory. On physical examination, her abdomen was markedly distended.
CT scan revealed an 18×21 cm sized unilocular cyst with irregular wall thickening and solid component at right adnexa (Fig. 1). Neither enlarged regional lymph node nor ascites was found. Hydronephrosis of right kidney and multiple uterine myomas were found. Laboratory analyses showed normal blood counts and normal serum value of CA-125. But, slightly elevated level of serum CA 19-9 was found (37.05 U/mL). Gastroscopy and colonoscopy showed no abnormality.
So, right ovarian neoplasm was suspected and an exploratory laparotomy was performed. A huge right retroperitoneal cystic tumor originating from right paracolic gutter was found. Although the tumor was close to the right ovary and appendix, there was no direct connection between them (Fig. 2). Small nodule was located in the right ovary and multiple uterine myomas were found. Left ovary and fallopian tube appeared normal. The cyst wall, having no apparent connection with any organs and showing no evidence of abdominal spread, was completely excised. Frozen section of the cystic tumor revealed a mucinous cystadenocarcinoma. Right salpingo-oophorectomy, washing cytology, myomectomy, lymphadenectomy, infracolic omentectomy, appendectomy and double J catheter insertion into right ureter were performed. The uterus and left ovary were preserved because the patient wished to remain fertile. Microscopically, no evidence of metastasis was found and no extracapsular invasion or vascular invasion was seen.
Following the surgery the patient recovered without any complication. She has been given no further treatment and 6 months after surgery, she has no evidence of recurrence.
Grossly, the mass was a well demarcated, unilocular, large cystic mass, measuring 19.0×16.0×9.0 cm. It was enveloped by a thick fibrous capsule and contained mucinous fluid. Inner surface showed variable-sized, grayish white, soft mural nodules (Fig. 3).
Microscopically, the tumor consisted of glands and papillary structures of columnar mucinous cells (Fig. 4A). The grade of cellular atypia was variable from gland-looking benign mucinous tumor area (Fig. 4B) to glands showing micropapillary architecture, nuclear stratification and atypia, compatible with borderline mucinous tumor (Fig. 4C) and glands of back-to-back arrangement, occasional cribriform pattern and marked nuclear atypia, compatible with well differentiated mucinous adenocarcinoma (Fig. 4D). By immunohistochemistry, the tumor cells were positive for cytokeratin 7, and focally positive for cytokeratin 20 and carcinoembryonic antigen. The Ki-67 index was up to 30%. Considering the clinical feature of the retroperitonium-confined single mass and the pathologic findings of transition from benign to malignant mucinous tumor, it was diagnosed as a primary retroperitoneal mucinous cystadenocarcinoma.
Retroperitoneal tumors of epithelial origin are extremely rare, because no epithelial cells are found in this area. Similar to mucinous tumors of the ovary, these neoplasms are divided into three categories: mucinous cystadenomas, mucinous borderline tumors (tumors of low malignant potential), and mucinous cystadenocarcinomas. Primary retroperitoneal mucinous cystadenocarcinoma was first reported by Roth and Ehrlich [3], in 1977. Although the origin of retroperitoneal mucinous cystadenocarcinomas is not clearly understood, proposed hypotheses include 1) origin from ectopic ovarian tissue [3,4], 2) retroperitoneal primary monodermal teratoma originating from displaced germ cells [5], 3) intestinal duplication, also known as enterogenous genesis [6,7], and 4) coelomic metaplasia [8,9]. To date, the hypothesis that has gained increasing support is coelomic metaplasia, that is, retroperitoneal mucinous cystadenocarcinomas arise from invagination of the peritoneal mesothelium, with subsequent mucinous metaplasia. The ultrastructural findings and immunohistochemical observations support this hypothesis [10].
The age at diagnosis ranges from 17 to 86 years old and the most common complaint at presentation has been abdominal discomfort and a slow-growing pelvic or abdominal mass [7,11]. Ovarian neoplasm rather than retroperitoneal neoplasm was suspected in our patient. Usually, preoperative diagnosis of retroperitoneal neoplasm is difficult because of the non-specific symptoms and the scarce aid of imaging examinations. Although radiologic studies such as ultrasonography and CT scan clearly detect cystic masses in ovary or pelvic organs, diagnosis of retroperitoneal tumor is extremely difficult [7].
CA 19-9 was slightly elevated in our patient, but tumor markers are not very helpful in differentiating the exact origin of the lesion, because CA-125, CA 19-9 may or may not be elevated. Tumor markers help in detecting a recurrent tumor, as in ovarian neoplasm [11].
The treatment of primary retroperitoneal mucinous cystadenocarcinoma remains controversial and no evidence based management guidelines are available. Laparotomy and complete tumor excision should be the principal modality of treatment, but the question about the extent of the surgery still remains. Most authors treated it as ovarian mucinous cystadenocarcinoma with a standard staging procedure including extirpation of the tumor, total hysterectomy and bilateral salpingo-oophorectomy with or without lymphadenectomy [10]. Most of the follow-up results were excellent. For young ovarian cancer patients who desire a baby, more conservative surgery with preservation of the uterus or ovary may be feasible in a properly selected patient population. According to the last American College of Obstetrics and Gynecology and European Society for Medical Oncology guidelines, fertility-sparing surgery for young women with invasive epithelial ovarian cancer can be adopted for stage IA and non-clear cell histology grade 1 or 2 [12]. Law et al. [2] recommended conservative management should be offered for women with a primary retroperitoneal mucinous cystadenocarcinoma, who desire a baby. They treated a 35-year-old woman by excision of the tumor alone. The patient conceived spontaneously 10 months after initial surgery and had no recurrence 5 years postsurgery. Because our patient also wished to remain fertile and had no evidence of abdominal spread, we performed fertility sparing surgery.
Currently, there is no clear evidence showing the benefit of adjuvant chemotherapy to primary retroperitoneal mucinous cystadenocarcinoma patients. Adjuvant chemotherapy is beneficial when invasion to adjacent structure is evident. Another point in favor of adjuvant treatment is evidence that primary retroperitoneal mucinous cystadenocarcinomas and ovarian mucinous tumor have similar mechanisms in their histogenesis [10]. Patients with this neoplasm generally have a good prognosis after complete removal of the neoplasm [13]. In our patient, the tumor was completely excised and no evidence of metastasis was found. So, we did not perform any adjuvant therapy.
Primary retroperitoneal mucinous cystadenocarcinoma is an extremely rare tumor and the treatment remains controversial. Fertility-sparing surgery should be considered for women with this neoplasm, who wish to remain fertile.
Figures and Tables
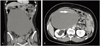 | Fig. 1Computed tomography scan. (A) Unilocular cyst with irregular wall thickening. (B) Solid component (arrow) in the cyst and hydronephosis of right kidney. |
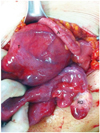 | Fig. 2Intraoperative photo showing the association of the tumor (T), appendix (A), and right ovary (RO). There was no direct connection between them. |
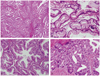 | Fig. 4Microscopic photography. (A) Low power view shows glands and papillae of mucinous cells (H&E, ×40). (B) Benign-looking area (H&E, ×200). (C) Borderline mucinous tumor-like area. Glands show micropapillary architecture and nuclear stratification (H&E, ×200). (D) Adenocarcinoma area. Glands are tightly arranged and show marked nuclear atypia (H&E, ×200). |
References
1. Kanayama T, Yoshino K, Enomoto T, Ohashi H, Fujita M, Ueda Y, et al. Primary retroperitoneal mucinous cystadenocarcinoma with mural nodules: a case report and literature review. Int J Clin Oncol. 2011. 09. 17. [Epub]. DOI: 10.1007/s10147-011-0313-4.
2. Law KS, Chang TM, Tung JN. Fertility-sparing treatment of a primary retroperitoneal mucinous cystadenocarcinoma. BJOG. 2006. 113:612–614.
3. Roth LM, Ehrlich CE. Mucinous cystadenocarcinoma of the retroperitoneum. Obstet Gynecol. 1977. 49:486–488.
4. Storch MP, Raghavan U. Mucinous cystadenocarcinoma of retroperitoneum. Conn Med. 1980. 44:140–141.
5. Peterson WF. Malignant degeneration of benign cystic teratomas of the overy: a collective review of the literature. Obstet Gynecol Surv. 1957. 12:793–830.
6. Chen JS, Lee WJ, Chang YJ, Wu MZ, Chiu KM. Laparoscopic resection of a primary retroperitoneal mucinous cystadenoma: report of a case. Surg Today. 1998. 28:343–345.
7. Matsubara M, Shiozawa T, Tachibana R, Hondo T, Osasda K, Kawaguchi K, et al. Primary retroperitoneal mucinous cystadenoma of borderline malignancy: a case report and review of the literature. Int J Gynecol Pathol. 2005. 24:218–223.
8. Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007. 302:389–398.
9. Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH, et al. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics. 2004. 24:1353–1365.
10. Tenti P, Carnevali L, Tateo S, Durola R. Primary mucinous cystoadenocarcinoma of the retroperitoneum: two cases. Gynecol Oncol. 1994. 55:308–312.
11. Tangjitgamol S, Manusirivithaya S, Sheanakul C, Leelahakorn S, Thawaramara T, Kaewpila N. Retroperitoneal mucinous cystadenocarcinoma: a case report and review of literature. Int J Gynecol Cancer. 2002. 12:403–408.
12. Satoh T, Hatae M, Watanabe Y, Yaegashi N, Ishiko O, Kodama S, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010. 28:1727–1732.
13. Suzuki S, Mishina T, Ishizuka D, Fukase M, Matsubara YI. Mucinous cystadenocarcinoma of the retroperitoneum: report of a case. Surg Today. 2001. 31:747–750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download