Abstract
Intraplacental choriocarcinoma is rare, and occasionally results in massive feto-maternal hemorrhage. We describe a case of an intraplacental choriocarcinoma diagnosed postpartum after a preterm cesarean delivery of a severely anemic newborn. The microscopic examination showed that clusters of malignant trophoblasts arose from residual normal chorionic villi and infiltrated into the intervillous spaces, confirmed as intraplacental choriocarcinoma. Fetomaternal hemorrhage is a rare complication of choriocarcinoma but its presence should always warrant detailed examination of placenta, mother, and infant.
Gestational trophoblastic disease encompasses a spectrum of interrelated tumors, including the hydatidiform moles, either partial or complete, to the more malignant forms, the invasive mole, the placental site and epithelioid trophoblastic tumors and choriocarcinoma [1]. Intraplacental choriocarcinoma, defined as choriocarcinoma in the placenta, is a rare variant of gestational choriocarcinoma accounting for no more than approximately 0.04% of gestational trophoblastic disease [2]. Older than 35 years or very young age of mother can be risk factors of gestational trophoblastic disease [3].
As fetomaternal hemorrhage can cause fetal distress and death, the possibility of intraplacental choriocarcinoma should be considered in these cases [4]. In this report, we present a case of an incidental intraplacental choriocarcinoma that was discovered when the placenta was examined in order to identify the cause of a newborn's anemia.
A 27-year-old woman, gravida 1, was referred to our institution for evaluation of fetal cardiomegaly and ascites at 31+4 weeks' gestation. Her past history was uneventful, and the results of antenatal laboratory tests and examinations were normal. The mother presented with decreased fetal movement from around 31 weeks of gestation. A transabdominal ultrasound scan with 3.5-5 MHz trasducers (Accuvix XQ, Medison, Seoul, Korea) revealed a severely dilatated heart, skin edema, pleural effusion and ascites, suggesting fetal hydrops. Cardiotocography was severely pathologic, showing a repeatitive late decerelation and decreased variability (Fig. 1). Emergency cesarean delivery was performed because of fetal hydrops and fetal distress. At delivery, the newborn baby weighed 2,020 g and had Apgar scores of 3 at 1 minute and 5 at 5 minutes. The baby was immediately intubated due to weak respiratory movement and was mechanically ventilated in the neonatal intensive care unit. He was markedly anemic and edematous. Initial chest X-ray showed a total white out pattern on the both lung fields. After admission, the baby's condition deteriorated progressively, with decreased arterial saturation. Sufficient oxygenation was not achieved on surfactant therapy and high-frequency oscillatory ventilation. Nitric oxide (NO) inhalation was applied because of continued hypoxemia. On laboratory tests, the hemoglobin concentration in the umbilical artery was 2.9 g/dL. He was treated with blood transfusion. Echocardiography showed severe distention of the right side of the heart, tricuspid valve regurgitation and patent ductus arteriosus with bidirectional shunt in accordance with persistent pulmonary hypertension of the newborn. Ultrasonographic scan of the brain showed cerebral infarction on left parietal lobe. At 4 days of age, he developed increasing respiratory distress and hypoxemia. In spite of the ventilator therapy with NO inhalation, blood transfusion and inotropic agents, he expired. Therefore fetomaternal hemorrhage was suspected. To clarify the mechanism of this fetomaternal hemorrhage, the placenta was examined, the pathological diagnosis of intraplacental choriocarcinoma was made.
The mother was promptly examined because of the risk of metastasis. A detailed physical examination was unremarkable. Chest X-ray, computed tomography (CT) scan of brain, chest, abdomen and pelvis revealed no metastatic lesion. Serial serum concentrations of human chorionic gonadotropin (hCG) were measured, for the once a week until normalization of serum hCG level. On the 18th day postpartum the hCG was 195 IU/L dropping to 2.54 IU/L at 58 days postpartum. Since there were no signs of dissemination, the mother was not treated with chemotherapy.
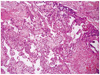
The fresh placenta weighed 691.4 g in weight and 22.6 × 17.2 × 2.8 cm in dimensions. There were several foci of hemorrhage and early thrombus (about 10% of total disk volume) and infarction (about 3%). Adjacent to a hemorrhagic focus that was at the central portion of the disk showed an ill-defined brown to gray colored friable lesion, measuring about 2 × 1 cm in size (Fig. 2). Microscopically, the placental disk was immature; decreased number of terminal villi and increased composition of intermediate villi with increased stroma. There were several histologic findings that suggest feto-materal hemorrhage; segmental infarct (arrow, A), intervillus hemorrhage (arrowhead, A) and increased number of nucleated red blood cells (RBCs) (white arrow, B) within intervillus hematoma (Fig. 3). The ill-defined lesion that had been grossly shown was composed of pleomorphic trophoblasts-cytotrophoblasts and syncytiotrophoblasts. Clusters of malignant trophoblasts surrounded the residual non-molar chorionic villi, and invaded into the intervillus spaces (Fig. 4). Tumor necrosis is present at center and periphery. Emboli or extension of the tumor into maternal surface were not found.
In 1963, Driscoll [5] first reported the "incidental finding" of a choriocarcinoma within a term placenta, without maternal or infantile metastasis. The term "choriocarcinoma in situ, within the placenta" was first introduced. However, this term was not accurate, since it indicated that the tumor was not capable of distant metastasis. The term "intraplacental choriocarcinoma" was recommended in the latter cases.
The prevalence of choriocarcinoma is low, but it is most often seen in women less than 15 years or more than 45 years of age, as well as in multiparous women [3,4,6]. However, due to the fact that the intra-placental lesion is usually small and can be easily missed, the true incidence of intraplacental choriocarcinoma can be higher than documented.
Irregular vaginal bleeding due to metastasis of uterus or vagina was often misconceived as abortion or placenta previa. Cough or hemoptysis due to pulmonary metastasis was often misdiagnosed as pulmonary infection or tuberculosis [7]. Intraplacental choriocarcinoma should always be suspected in the third trimester or at term when symptoms related to metastasis occurred. Detailed examination, such as physical examination, serum β-hCG, X-ray, ultrasound, CT scan and MRI should be performed to rule out metastasis when intraplacental choriocarcinoma is suspected. In our case, the mother had not any clinical symptom related to metastasis. This tumor is always detected as infarct-like lesion on gross examination. Liu and Guo [8] reviewed 21 cases in the articles and they said "different findings have been observed during the process of gross examination of the placenta". In the cases which described the mass as a single lesion, the tumor presented as tan to yellow and circumscribed nodules, ranging from 0.5 to 5 cm in diameter.
One case showed more than 50 white small nodules (<0.5 cm) scattered throughout the placenta [9]. In other case with no gross lesion, pathologists had to examine entire placenta to find the microscopic foci [10]. Our case showed a single nodule as brown to gray colored friable lesion in mid-portion of the placental disk and measures about 2 × 1 cm in size. Microscopically, the malignant trophoblasts growing about the residual villi are the typical pattern of intraplacental choriocarcinoma. On the other hand, any non-molar villi is not present in the case of intrauterine choriocarcinoma. The malignant trophoblastic cells showed the characteristic biphasic pattern of cytotrophoblast and syncytiotrophoblast, with marked pleomorphism, prominent nucleoli and frequent mitoses [8]. Jacques et al. [11] described that intraplacental choriocarcinoma represents a primary malignancy arising from the trophoblast of the placenta. And they said it is supported by the histologic finding of malignant trophoblasts transition from benign trophoblast [11].
Presented case showed peritumoral hemorrhage, multifocal intervillus hemorrhage, and early thrombus. And increased numbers of nucleated RBCs are founded within thrombus, intervillus hemorrhagic spaces, and fetal vessels in chorionic villus. Those histologic findings suggest chronic anemia of fetus. Maternal blood sampling was not carried out, a quantitative measurement about the number of nucleated RBC have not be carried out. Chronic fetomaternal hemorrhage that is one of the causes of non-immune fetal hydrops shows variable histological changes as well as intervillus hemorrhage and thrombus. Macroscopically, placenta is not markedly enlarged as in thalassemia, but extremely pale. Multiple placental infarct can be seen. Microscopically, nucleated RBCs are increased in the fetal circulation. Hemosiderin is occasionally seen within chorionic macrophages. But, it is difficult to differentiated fetomaternal hemorrhage from other causes of non-immune fetal hydrops; congenital anomaly, aplastic anemia, Parvovirus infection, thalassemia, etc. by histological finding only.
In 1962, Benson et al. [12] were the first to describe a case of massive fetomaternal hemorrhage as a complication of choriocarcinoma. The complications of intraplacental choriocarcinoma include severe fetomaternal hemorrhage, retroplacental hemorrhage, placental abruption and fetal hydrops. These complications often lead to fetal distress, fetal anemia, intrauterine growth restriction, intrauterine fetal death (IUFD) and stillbirth [8,13]. The correlation and the pathogenesis mechanism are still unknown.
Treatment of choriocarcinoma with localized intraplacental lesions and no evidence of metastasis is controversial. Santamaria et al. [7] described a pregnancy resulting in IUFD with evidence of fetal-maternal hemorrhage. The extensive workup of mother for metastatic choriocarcinoma was negative, but methotrexate was given empirically to prevent metastasis [8]. However, Duleba et al. [14] suggested that patients with choriocarcinoma confined to the placenta , with rapid decrease of serum β-hCG postpartum, do not need chemotherapy. However, the mother and the infant need extensive workup and serial serum β-hCG monitoring to rule out metastasis, as well as long-term close follow-up. In our case, there were no signs of dissemination and rapid decrease of serum β-hCG, the mother was not treated with chemotherapy.
This case clearly illustrates the importance of clinico-pathological correlation and fetomaternal hemorrhage should always be followed by detailed examination of the placenta as well as serial serum-hCG monitoring, in an attempt to diagnose possible intraplacental choriocarcinoma.
Figures and Tables
Fig. 1
Cardiotocographic monitoring before delivery shows repetitive late decelerations and decreased variability.
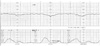
Fig. 2
The cut surface of placental disk is pale but not hydroptic. Under the cord insertion site, an ill-defined brown to gray lesion is present (white arrow) and the background area is hemorrhagic.

References
1. Shih IeM. Gestational trophoblastic neoplasia--pathogenesis and potential therapeutic targets. Lancet Oncol. 2007. 8:642–650.
2. Sebire NJ, Lindsay I, Fisher RA, Seckl MJ. Intraplacental choriocarcinoma: experience from a tertiary referral center and relationship with infantile choriocarcinoma. Fetal Pediatr Pathol. 2005. 24:21–29.
3. Sebire NJ, Foskett M, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG. 2002. 109:99–102.
4. Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer. 1976. 38:1373–1385.
5. Driscoll SG. Choriocarcinoma: An "incidental finding" within a term placenta. Obstet Gynecol. 1963. 21:96–101.
6. Berkowitz RS, Goldstein DP, Bernstein MR. Choriocarcinoma following term gestation. Gynecol Oncol. 1984. 17:52–57.
7. Santamaria M, Benirschke K, Carpenter PM, Baldwin VJ, Pritchard JA. Transplacental hemorrhage associated with placental neoplasms. Pediatr Pathol. 1987. 7:601–615.
8. Liu J, Guo L. Intraplacental choriocarcinoma in a term placenta with both maternal and infantile metastases: a case report and review of the literature. Gynecol Oncol. 2006. 103:1147–1151.
9. Lage JM, Roberts DJ. Choriocarcinoma in a term placenta: pathologic diagnosis of tumor in an asymptomatic patient with metastatic disease. Int J Gynecol Pathol. 1993. 12:80–85.
10. Tsukamoto N, Kashimura Y, Sano M, Saito T, Kanda S, Taki I. Choriocarcinoma occurring within the normal placenta with breast metastasis. Gynecol Oncol. 1981. 11:348–363.
11. Jacques SM, Qureshi F, Doss BJ, Munkarah A. Intraplacental choriocarcinoma associated with viable pregnancy: pathologic features and implications for the mother and infant. Pediatr Dev Pathol. 1998. 1:380–387.
12. Benson PF, Goldsmith KL, Rankin GL. Massive foetal haemorrhage into maternal circulation as a complication of choriocarcinoma. Br Med J. 1962. 1:841–842.
13. Takai N, Miyazaki T, Yoshimatsu J, Moriuchi A, Miyakawa I. Intraplacental choriocarcinoma with fetomaternal transfusion. Pathol Int. 2000. 50:258–261.
14. Duleba AJ, Miller D, Taylor G, Effer S. Expectant management of choriocarcinoma limited to placenta. Gynecol Oncol. 1992. 44:277–280.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download