Abstract
Objective
To assess the incidence of ectopic pregnancy (EP) in St. Luke's Hospital, Anua in Uyo metropolis, Nigeria. Data on EP incidence in developing countries are rare and often out of date, particularly in Nigeria.
Methods
A five-year retrospective study (2000-2004) was carried out, examining all cases of EP registered in the medical files of records in the casualty, maternity and surgical departments of St. Luke's Hospital, Anua in Uyo metropolis, which before the advent of the University of Uyo Teaching Hospital, in 2002, was the hub of medical activities in Akwa Ibom State.
Results
Within the period under study, 2,3951 pregnancies was registered in that hospital out of which 72 cases of ectopic pregnancies was reported (3/1,000 pregnancies). Most of the affected females were young single women and students with 81.9% of them between 21 and 30 years of age. Mortality was 1.4% in the study. Related risk factors included pelvic inflammatory disease, previous history of abortions, infertility and a previous history of EP. These problems are compounded by social issues leading to multiple sexual partners and financial stress resulting from the palpable poverty in Nigeria today.
Conclusion
Reports of hospital-based incidence of EP in various centers across Nigeria varies but has increased over the last decade. Health professionals and public health officials in developing countries, especially those in Africa, should consider EP as a major obstetric problem for maternal morbidity. Early detection and more public education as well as advocacy programs targeted at women are needed to solve the problem of EP in Uyo, Akwa Ibom state, Nigeria.
Ectopic pregnancy is defined as a pregnancy in which the implantation of the embryo occurs outside the uterine cavity, most frequently in one of the two fallopian tubes or, more rarely, in the abdominal cavity [1]. During the first three months of pregnancy, ectopic pregnancy is the leading cause of maternal morbidity and mortality [2,3] in industrialized countries, and possibly the second most frequent cause in developing countries (after abortion complications) [1]. It is a common obstetric problem the world over.
Though the global incidence has been rising during the last three decades [3,4], the incidence of the condition varies from country to country depending on the risk factors predominant in the geographical region. In most of Europe and North America, the incidence of ectopic pregnancy has tripled over the last 30 years [1]. Although hospital-based African studies indicate ectopic pregnancy incidence has probably increased in Africa in recent decades, major methodological limitations in the published literature make it impossible to draw formal conclusions concerning the incidence of ectopic pregnancy in Africa in recent years. Case fatality rates of around 1%-3%, 10 times higher than that reported in industrialized countries have been reported [5].
The trend is even worse in Nigeria where women present late with ruptured ectopy in more than 80% of the cases [6,7]. In a study in Ile-Ife teaching hospital, a hospital-based incidence of ectopic pregnancy quadrupled between 1977 and 1987 (from 0.4% to 1.7%) [1]. Also in Lagos, it is responsible for 8.6% of maternal deaths, and has a case fatality rate of 3.7% [8]. In Jos, a total of 168 ectopic pregnancies were managed and 9,638 deliveries occurred during the same period. This gives a prevalence rate of 1.74% [9]. This trend was also observed in a retrospective study of trends in ectopic pregnancy in Ilorin-Nigeria by Aboyeji et al. [10], where there observed that ectopic pregnancy was 1 in 69 deliveries (1.4%) at the University of Ilorin Teaching Hospital. This represented a one and half increase from survey conducted between 1987 and 1991 which put the incidence at 0.9%.
We are challenged by poor diagnostic tools, limited capacity to handle emergencies and consequent burden of increased maternal morbidity and mortality with consequent reproductive failure [11]. In Lagos it has been reported to be responsible for 30% of emergency gynecologic admissions [12], 8.6% of maternal deaths and has a case fatality rate of 3.7% [13]. In Ghana, a neighbouring West African country, a case fatality rate of 29.9/1,000 was reported [14]. The reported etiologic factors for ectopic pregnancy include pelvic inflammatory disease (PID), post-abortal sepsis, post-partum sepsis, previous ectopic pregnancy, reversal of previous tubal pregnancy, previous Caesarean section, tubal spasm, congenital defects of the fallopian tube, and psychological and emotional factors [15]. Early age at first sexual intercourse is a significant risk factor [8,15,16]. Adolescents are known to be very prone to sexually transmitted diseases (STDs) and the reason for this is both behavioral and biological [8]. Behaviorally, adolescents tend to choose partners who are likely at risk of STD [16].
The treatment of ectopic pregnancy is influenced by the clinical state of the patient, site of the ectopic gestation, the reproductive wish of the patient as well as availability of facilities and technological expertise. However, surgical treatment still remains the norm [11]. Ectopic pregnancy can be treated surgically or nonsurgically depending if it is ruptured or not. Due to advances in the diagnostic techniques, it has become possible to identify and manage ectopic pregnancy before they cause clinical symptoms in many developed countries [17]. This is not so in most developing countries like Nigeria. The management of ectopic pregnancy has been improved upon by the use of ultrasound, laparoscopy and monitoring of the beta- human chorionic gonadotrophin [18]. Early diagnosis before tubal rupture is important in reducing mortality as well as preserving the potential for future fertility through conservative management [19].
Fertility is substantially improved when conservative surgery is utilized instead of salpingectomy. Subsequent intrauterine pregnancy rates have been found to be 76% when conservative surgery is performed and 44% when salpingectomy is performed [20]. The aim of this review therefore is to determine the incidence and trend of ectopic pregnancy, associated aetiological factors and make suggestions on preventive mechanisms at the St. Luke's Hospital, Anua, Uyo.
This was a retrospective study of ectopic pregnancies at St. Luke's Specialist Hospital, Anua, Uyo in South-South geopolitical zone of Nigeria between January 2000 and December 2004. The case notes/medical records of the patients from were examined for reported cases of ectopic pregnancies. The search spanned the casualty department, the theatre, and maternity sections of the hospital. Theatre registers were screened for those that underwent surgeries, the operation notes were scanned and data collated. Records of all registered pregnancies for the same period were obtained from the maternity section of the hospital. Information on the biosocial data, clinical symptoms and signs, sites and treatment options, risk factors for the disease as well as associated morbidity were extracted. The data was analyzed with simple descriptive statistics and presented in the tables under results section.
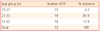
In the five-year review, there were a total of 23,981 deliveries and 72 ectopic gestations recorded. This gives an incidence of 0.30% of total deliveries with highest incidence recorded in 2004 (0.46%) and lowest in 2003 (0.20%). The majority of ectopic gestations, 59 (81.9%) were in the 21-30 age group (Table 1). Single women accounted for 51.4% of all cases with majority of them students (52.8%) (Table 2). Table 3 also shows that the highest yearly incidence of ectopic gestation was recorded in 2004 (0.46%) as against 0.20% in 2003. Thirty seven (51.4%) of the ectopic pregnancy occurred in the right fallopian tube, 23 (31.9%) in the left fallopian tube, 6 (8.3%) in the cornua, 4 (5.6%) in the isthmus while 2 (2.8%) in the ampullary region (Table 4).The three most common risk factors in the study are: pelvic inflammatory disease 37 (51.4%), previous abortions 18 (25.0%), and infertility 2 (2.8%) (Table 5).
Mortality: 1 patient was reported dead in the sampled period which gives a 1.4% mortality rate in this survey.
Ectopic pregnancy is prevalent in our environment affecting mainly young women of low parity who desire future pregnancies. The subsequent impact on future fertility of these women could be improved if efforts are focused on early diagnosis to prevent tubal rupture. Early diagnosis prior to rupture offers opportunity for medical management and conservative surgical procedures that are proven to improve future fertility [9]. The incidence of ectopic pregnancy has followed an increasing trend during the last three decades throughout the world [4,16]. The incidence of 0.3% or 3 per 1,000 in our present study is lower than most reported cases in the country. Musa et al. [9] reported an incidence of 1.74% in Jos, Anorlu et al. [8] reported an incidence of 23.1/1,000 in Lagos, Udigwe et al. [11] reported an incidence of 1.3% in Nnewi, Aboyeji et al. [10] reported 1.4% incidence in Ilorin. We believe that gross underreporting of cases and patronage of traditional birth attendants and prayer houses instead of hospitals on health issues and pregnancy related complications by patients might have be contributory. The incidence of 0.3% is comparable to similar studies in India which recorded 0.4% [21].
Mortality rate of 1.4% we reported was also lower compared to the 8.6% reported in Lagos by Anorlu et al. [8] and Amoko and Buga [17] which recorded 2.0% in a South African population. But this was similar to that reported by Igbarese et al. [6] which was around 1.5%. Mortality depends on population size as well as the clinical state of the patient on presentation [11]. In our case, we adduce that this was as a result of prompt surgical intervention in the hospital managed by a European medical missionaries during the period under study.
The peak age incidence was amongst women in the age group of 21-30 years which corroborates with findings of Majhi et al. [3] and Igbarese et al. [6]. This corresponds to the age of reproduction and peak sexual activity. Also, the incidence in present study was higher amongst single women and students than married women. This can be attributed to the fact that they are more prone to STDs as a result of having multiple sexual partners and they usually resort to induced abortion (which is usually performed by non-physicians resulting in complications and late presentation) from unwanted pregnancies. Patients are also unlikely to seek proper medical attention and resort to buying of drugs from patent medicine stores with the resultant effect of poor treatment leading to tubal blockage/damage [10] thus PID. Patients with ectopic pregnancy were found of a lower parity in some descriptive studies from Nigeria [12,22]. However, recent studies have shown a significantly increased risk of EP in women aged 40 and over 40 years [23,24]. Cumulative risk factors associated with getting pregnant at an older age were cited as being responsible for this increased risk of EP with age.
From the results, it has become clear that a sizeable percentage of our young women in their prime of reproductive years have become subjected to emotional and psychological problems of failure of reproduction associated with ectopic pregnancy in our environment where a lot of attention is placed on child bearing.
Due to advances in diagnostic techniques, it has become possible to identify and manage ectopic pregnancies before they cause clinical symptoms in many developed countries. However, the situation appears not to be the same in developing countries [17]. Early detection and conservative management using transvaginal sonography, laparoscopy and laparoscopic surgeries are the hallmark of treatment in developed countries [25,26]. But in developing countries 95% to 100% of the patients present late, with ruptured ectopics that are either immediately clinically obvious or where culdocentesis is virtually diagnostic because of haemoperitoreum. In these cases, the only option available was open laparotomy and salpingectomy [27]. The emphasis in the management of EP is on early diagnosis before rupture and conservative surgery.
However, in most developing countries especially Nigeria where patients still present late after rupture, salpingectomy remains the operative procedure [28]. Salpingectomy is the commonest surgical management for tubal pregnancy in Nigeria because most of the women present late [7,29]. Incidentally, this procedure which leads to tubal loss and reduced reproductive potentials is the commonest management option in low resource settings [30]. Intrauterine pregnancy rate after salpingectomy is about 45% with a 9% repeat EP [30]. In salpingostomy, tissue handling is minimized to reduce tissue trauma and prevent tubal occlusion or peritubal adhesions. The success of reconstructive tubal surgery for EP can be only measured in terms of subsequent live births the individual achieves. During the surgical treatment of EP by both laparatomy and laparoscopy, the state of the contra-lateral tube is noted. The condition of the contra-lateral tube has been reported to play a crucial role in subsequent fertility of patients with EP [31,32].
Most of the patients in our report had ampullary ectopic pregnancy (83.3%) which is consistent with studies from other centers [33-35]. The preponderance of ectopic pregnancies on the right Fallopian tube (51.4%) is similar to trends all over the world, with incidence of up to 66.2% and the left 33.8% of the cases [7,9,36]. Nordenskjöld and Ahlgren [36] attributed this right-sided preponderance to appendicitis.
As in industrialized countries, PID associated with STDs must be considered as the most important risk factor for ectopic pregnancy in developing countries [5]. About 86% of the cases reported by Amoko and Buga [17] had evidence of previous pelvic infection, thus making PID the most important risk factor for ectopic pregnancy. Our research findings are also in agreement with these findings and other [8,11] thus implicating PID as a major risk factor for ectopic pregnancy. Other factors like previous history of abortion and history of previous ectopic gestation were also predisposing factors for ectopic pregnancy [10,37,38].
While this study is a hospital-based one with its inherent biases, we would expect that population-based (cohort studies) of this magnitude be conducted to evaluate the actual incidence of ectopic pregnancy in the population as this would give better trends. In the light of the results obtained in this study and the body of existing literatures about ectopic pregnancy in developing countries like ours, emphasis should be placed on primary prevention and early detection of ectopic pregnancy. The burden of infections as a leading risk factor for ectopic pregnancy can be addressed by a more proactive approach on the professional and health care officials' approach to issues of maternal health.
Ectopic pregnancy, a gynecological emergency, is prevalent in our environment affecting mainly young women of low parity who desire future pregnancies. The subsequent impact on future fertility of these women could be very much improved if efforts were focused on primary prevention and early diagnosis to prevent tubal rupture. Advocacy to improve the knowledge of young women on risk factors and the symptoms of ectopic pregnancy should be encouraged. This would facilitate the early reporting to hospital for diagnoses and conservative management. We suggest also that our hospitals should be adequately equipped to handle early diagnoses and prompt treatment to reduce the incidence, morbidity, mortality and infertility associated with ectopic gestation.
Figures and Tables
References
1. Thonneau P, Hijazi Y, Goyaux N, Calvez T, Keita N. Ectopic pregnancy in Conakry, Guinea. Bull World Health Organ. 2002. 80:365–370.
2. Okunlola MA, Adesina OA, Adekunle AO. Repeat ipsilateral ectopic gestation: a series of 3 cases. Afr J Med Med Sci. 2006. 35:173–175.
3. Majhi AK, Roy N, Karmakar KS, Banerjee PK. Ectopic pregnancy: an analysis of 180 cases. J Indian Med Assoc. 2007. 105:308–312.
4. Rajkhowa M, Glass MR, Rutherford AJ, Balen AH, Sharma V, Cuckle HS. Trends in the incidence of ectopic pregnancy in England and Wales from 1966 to 1996. BJOG. 2000. 107:369–374.
5. Goyaux N, Leke R, Keita N, Thonneau P. Ectopic pregnancy in African developing countries. Acta Obstet Gynecol Scand. 2003. 82:305–312.
6. Igberase GO, Ebeigbe PN, Igbekoyi OF, Ajufoh BI. Ectopic pregnancy: an 11-year review in a tertiary centre in the Niger Delta. Trop Doct. 2005. 35:175–177.
7. Gharoro EP, Igbafe AA. Ectopic pregnancy revisited in Benin City, Nigeria: analysis of 152 cases. Acta Obstet Gynecol Scand. 2002. 81:1139–1143.
8. Anorlu RI, Oluwole A, Abudu OO, Adebajo S. Risk factors for ectopic pregnancy in Lagos, Nigeria. Acta Obstet Gynecol Scand. 2005. 84:184–188.
9. Musa J, Daru PH, Mutihir JT, Ujah IA. Ectopic pregnancy in Jos Northern Nigeria: prevalence and impact on subsequent fertility. Niger J Med. 2009. 18:35–38.
10. Aboyeji AP, Fawole AA, Ijaiya MA. Trends in ectopic pregnancy in Ilorin, Nigeria. Nigerian J Surg Res. 2002. 4:6–11.
11. Udigwe GO, Umeononihu OS, Mbachu II. Ectopic pregnancy: a 5 year review of cases at Nnamdi Azikiwe Universty Teaching Hospital Nnewi. Niger Med J. 2010. 51:160–163.
12. Abudu OO, Egwatu JI, Imosemi OO, Ola ER. Ectopic pregnancy: Lagos University Teaching Hospital experience over a five year period. Nig Q J Hosp Med. 1999. 9:100–103.
13. Abudu OO, Olatunji AD. A review of maternal mortality in Lagos University Teaching Hospital. Niger Med Pract. 1996. 31:12–16.
14. Baffoe S, Nkyekyer K. Ectopic pregnancy in Korle Bu Teaching Hospital, Ghana: a three-year review. Trop Doct. 1999. 29:18–22.
15. Doyle MB, DeCherney AH, Diamond MP. Epidemiology and etiology of ectopic pregnancy. Obstet Gynecol Clin North Am. 1991. 18:1–17.
16. Aral SO. Germaine A, Holmes KK, Piot P, Wasserheit JN, editors. Sexual behaviour as risk factor for sexually transmitted diseases. Reproductive tract infections: global impact and priorities for women's reproductive Health. 1992. New York: Plenum Press;185–191.
17. Amoko DH, Buga GA. Clinical presentation of ectopic pregnancy in Transkei, South Africa. East Afr Med J. 1995. 72:770–773.
18. Gracia CR, Barnhart KT. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001. 97:464–470.
19. Gazvani MR. Modern management of ectopic pregnancy. Br J Hosp Med. 1996. 56:597–599.
20. Sherman D, Langer R, Sadovsky G, Bukovsky I, Caspi E. Improved fertility following ectopic pregnancy. Fertil Steril. 1982. 37:497–502.
21. Indian Council of Medical Research Task Force Project. Multicentre case-control study of ectopic pregnancy in India. J Gynaecol Obstet India. 1990. 40:425–430.
22. Ilesanmi AO, Sobowale OA. Ectopic pregnancy in Ibadan, Nigeria. Niger Med J. 1992. 23:11–14.
23. Simms I, Rogers PA, Nicoll A. The influence of demographic change and cumulative risk of pelvic inflammatory disease on the incidence of ectopic pregnancy. Epidemiol Infect. 1997. 119:49–52.
24. Storeide O, Veholmen M, Eide M, Bergsjø P, Sandvei R. The incidence of ectopic pregnancy in Hordaland County, Norway 1976-1993. Acta Obstet Gynecol Scand. 1997. 76:345–349.
25. Odejinmi FO, Rizzuto MI, Macrae RE, Thakur V. Changing trends in the laparoscopic management of ectopic pregnancy in a London district general hospital: 7-years experience. J Obstet Gynaecol. 2008. 28:614–617.
26. Karri K, Harris CP. Successful laparoscopic management of ectopic pregnancy in a district general hospital. J Obstet Gynaecol. 2005. 25:769–771.
27. Tulandi T, Saleh A. Surgical management of ectopic pregnancy. Clin Obstet Gynaecol. 1997. 42:31–35.
28. Ibekwe PC. Ruptured advanced tubal ectopic pregnancy simulating uterine rupture: a case report. Niger J Med. 2004. 13:196–198.
29. Egwuatu VE, Ozumba BC. Unexpectedly low ratio and falling incidence rate of ectopic pregnancy in Enugu, Nigeria, 1978-1981. Int J Fertil. 1987. 32:113–115.
30. Eze JN. Successful intrauterine pregnancy following salpingostomy; case report. Niger J Med. 2008. 17:360–362.
31. Kjellberg L, Lalos A, Lalos O. Reproductive outcome after surgical treatment of ectopic pregnancy. Gynecol Obstet Invest. 2000. 49:227–230.
32. Tuomivaara L, Kauppila A. Radical or conservative surgery for ectopic pregnancy? A follow-up study of fertility of 323 patients. Fertil Steril. 1988. 50:580–583.
33. Oronsaye AU, Odiase GI. Incidence of ectopic pregnancy in Benin City, Nigeria. Trop Doct. 1981. 11:160–163.
34. Swende TZ, Jogo AA. Ruptured tubal pregnancy in Makurdi, north central Nigeria. Niger J Med. 2008. 17:75–77.
35. Sy T, Diallo Y, Toure A, Diallo FB, Balde AA, Hyjazi Y, et al. Management of ectopic pregnancy in Conakry, Guinea. Med Trop (Mars). 2009. 69:565–568.
36. Nordenskjöld F, Ahlgren M. Risk factors in ectopic pregnancy. Results of a population-based case-control study. Acta Obstet Gynecol Scand. 1991. 70:575–579.
37. Atrash HK, Strauss LT, Kendrick JS, Skjeldestad FE, Ahn YW. The relation between induced abortion and ectopic pregnancy. Obstet Gynecol. 1997. 89:512–518.
38. Skjeldestad FE, Gargiullo PM, Kendrick JS. Multiple induced abortions as risk factor for ectopic pregnancy. A prospective study. Acta Obstet Gynecol Scand. 1997. 76:691–696.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download