Abstract
Ovarian pregnancy is a rare form of ectopic pregnancy, and is most difficult to diagnosis before surgery. This study was conducted as a retrospective cohort series of women presenting to Korea University Medical Center between January 2000 and July 2011 in whom a diagnosis of ovarian pregnancy was made. We had total 7 cases of ovarian pregnancies between January 2000 and July 2011 at Korea University Medical Center. The mean age of the 7 ovarian pregnancy cases was 33.0 years (range, 22 to 42 years), mean parity was 1.0 (range, 0 to 3), mean gestational age at diagnosis was 8.0 (range, 5.6 to 12.4 weeks), and mean initial beta human chorionic gonadotropin (HCG) was 1,195.49 mIU/mL (range, 200.5 to 2,098.201 mIU/mL). The beta HCG level was lower remarkably in ovarian pregnancy group than in the tubal pregnancy group, and further study may be needed to find out the correlation between beta HCG level and ovarian pregnancy.
Primary ovarian pregnancy is a rare form of ectopic gestation occurring about 1 in 7,000 to 1 in 40,000 deliveries. And accounts for 1% of all ectopic pregnancies. In our department, for last ten years, we had total 653 cases of ectopic pregnancies(include tubal, ovarian, cornual pregnancies.) and there was total 7 cases of ovarian pregnancies, which accounts for 1% of all ectopic pregnancies [1]. The diagnostic criteria were described by Spiegelberg [2] as follow: 1) the fallopian tube on the affected side must be intact; 2) the gestational sac must occupy the same position as the ovary; 3) the ovary must be connected to the uterus by the utero-ovarian ligament; and 4) ovarian tissue must be located in the gestational sac wall. The diagnosis of the ovarian pregnancy can be made by laparotomy or laparoscopic surgery and confirmed by post operation pathologic results. We have experienced seven cases of ovarian pregnancy, which are presented with a brief review of the literature.
This study was conducted as a retrospective cohort series of women presenting to Korea University Medical Center between January 2000 and July 2011 in whom a diagnosis of ovarian pregnancy was made. Cases were identified by reviewing the computerized diagnoses of all pregnant women who were patients underwent surgery and the diagnosis was pathologically confirmed. Comprehensive clinical and laboratory information were extracted from medical and obstetric records of all identified cases. Also data of women was acquired between January 2010 and July 2011 women whom a diagnosis of tubal pregnancy was made.
The average beta human chorionic gonadotropin (HCG) levels are acquired and we compared between the two groups (ovarian pregnancy vs. tubal pregnancy).
We had total 7 cases of ovarian pregnancies between January 2000 and July 2011 and 40 cases of tubal pregnancies between January 2010 and July 2011 at Korea University Medical Center. Among these seven cases of ovarian pregnancies were identified. The characteristics of study sample are noted in Table 1. The mean age of the 7 ovarian pregnancy cases was 33.0 years (range, 22 to 42 years), mean parity was 1.0 (range, 0 to 3), mean gestational age at diagnosis was 8.0 (range, 5.6 to 12.4 weeks), and mean initial beta HCG was 1195.49 mIU/mL (range, 200.5 to 2,098.201 mIU/mL). Case 1 was excluded in calculating mean beta HCG level, because initial beta HCG level was not measured before surgery. The lesion was found on right side in 3 patients, and left side in 4 patients. From the 7 ovarian pregnancy patients, we presented two recent cases (cases 6 and 7).
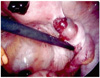
A 30-year-old women with primigravida who underwent intrauterine insemination on April 30, 2010 and showed no G-sac on ultrasonogram visited our clinic under impression of ectopic pregnancy. Initial beta-HCG was 2,098.2 mIU/mL. She complained mild abdominal pain and on ultrasound examination we found a mass containing a sac medial to the left ovary and it was highly suspicious of an ectopic pregnancy and there was no evidence of hemoperitoneum. She was hemodynamically stable, hemoglobulin level was 12.0 g/dL. Our initial treatment was intramuscular injection of methotrexate (1 mg/kg/day) followed by leucovorin (0.1 mg/kg/day) alternatively for eight days, and beta HCG follow up was done at third day (4,597.94 mIU/mL), fifth day (7,293.85 mIU/mL), one day after the course was finished (4,226.50 mIU/mL). Since beta-HCG showed decreased levels, and patient did not complain abdominal pain anymore she was planned to discharge. But she suddenly complained severe abdominal pain. Transvaginal ultrasound scan was taken and 4.0×0.5 cm fluid collection in anterior side of uterus. We decided to undergo laparoscopic surgery. About 200 mL hemartoma was seen in post cul-de-sac, and the uterus, both tubes and right ovary was intact. A left ovarian 3 cm sized hemorrhagic mass was seen with actively bleeding and wedge resection of left was ovary was done (Fig. 1). On pathological findings the ovarian mass showed products of conception (Fig. 2). Postoperative day 3 quantitative beta hexachloro-cyclohexane (HCH) was 370.06 mIU/mL, day 5; 286.98 mIU/mL, day 12; 20.51 mIU/mL.
A 39-year-old para 1, at 6 + 2/7 weeks' estimated gestational age by last normal menstrual period complained whole abdominal pain and visited local gynecologic clinic and underwent ultrasonogram. Under impression of ruptured ectopic pregnancy combined with hemoperitoneum she was referred to our hospital by emergency room, complaining severe low abdominal pain and tenderness. Initial hemoglobulin was 10.8 g/dL, quantitive beta-HCG of 3,662.01 mIU/mL. Transvaginal ultrasound showed 6.9 × 4.2 cm hematoma shadow in posterior cul-de-sac, and 6.3 × 1.0 cm fluid collection in uterovesicular pouch, both adnexas were not defined well, and uterus showed normal appearance with 6mm endometrial stripe. Initial impression was rupture of ectopic pregnancy. But since we could not detect ectopic mass in ultrasonogram, there were possibilities of intrauterine pregnancies, or hemorrhagic ovarian cyst rupture which also could lead to hemoperitoneum. Considering the amount of blood filled in pelvic cavity, and development of an acute abdomen, surgical treatment was required.
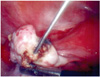
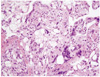
At laparoscopy, about 500 mL of blood was found in posterior cul do sac. Both fallopian tube appeared normal. After inspection we found 1 cm sized cyst-like mass on the surface of right ovary with bleeding (Fig. 3) and ovarian mass excision was done. Pathology confirmed a right ovarian gestation, demonstrating ovarian tissue histologically present in the wall of trophoblastic tissue of the gestational sac fulfilling the fourth of Spiegelberg;s criteria (Fig. 4). Postoperative day 3 quantitative beta HCH was 648.57 mIU/mL, day 5; 286.98 mIU/mL, day 11; 37.72 mIU/mL.
As mentioned above, ovarian pregnancy is a rare form of ectopic pregnancy in which the gestational sac is implanted in the ovary. There are several major events in the medical histories of the affected women that lead to the presumption of possible risk factors for ovarian pregnancy, and accompany the increase in prevalence in several decades. The main risk factors for ovarian pregnancies are endometriosis, former operations on the adnexa, former infectious diseases, previous reproductive system pathology or infertility, in vitro fertilization with embryo transfer, polycystic ovarian disease, and the use of an intrauterine device (IUD) [3]. However it is unclear whether the rise is due to true increase in prevalence or simply a result of emergence of better and earler diagnosis methods such as better availability of earlier quantitive beta-HCG measurements, early and more precise transvaginal sonogram, and early use of laparoscopy [4-6]. Risk factors for ovarian pregnancy are similar to tubal pregnancy of which concurrent use of and IUD seems important. In the past, ovarian pregnancy had been treated by ipsilateral oophorectomy, but the trend has since shifted toward conservative surgery such as cystectomy or wedge resection performed at either laparotomy or laparoscopy. Currently, laparoscopic surgery is the treatment of choice.
In case 6, medical management failed despite the fact that prerequisites predicting successful outcome with medical management were fulfilled. Medical management is offered in early unruptured ectopic pregnancies having serum beta-HCG<5,000-10,000 mIU/mL, no fetal cardiac activity, no fetal cardiac activity, and adnexal mass<3.5-4 cm. The known success rate of single-dose methotexate is 87% with 8% patients needing a second dose [7,8]. Some factors predicting failure of medical management with methotrexate are high serum beta-HCG level, high serum progesterone level, presence of cardiac activity [9], presence of yolk sac [10], high pretreatment folic acid levels [11], lack of side effects of methotrexate [12], and endometrial thickness [12]. In this case the patient had nearly all features that predict successful medical management; beta-HCG level initially (2,098.23 mIU/mL), no cardiac activity, endometrial thickness<12 mm, and no yolk sac. Although methotrexate is an effective therapeutic option for the management of unruptured ectopic pregnancy, the case demonstrates that it may fail despite the presence of factors predicting successful outcome. It is not clear whether methotrexate failure is more likely in the case of ovarian ectopic pregnancy than tubal ectopic pregnancies. Usually the ovary and the tube get their blood supply from similar sources which is branches of uterine and ovarian vessel. However ovarian pregnancy is usually located peripherally toward the peritoneal cavity, and this location may have a reduce blood flow, therefore, the amount of methotrexate delivered to it would be lower. Doppler blood flow assessment may be helpful to assess whether methotrexate treatment would be effective.
In case 7, as described above, the ectopic mass was not detected in ultrasonogram, but there was hemoperitoneum sign and severe abdominal pain, so emergency laparoscopic surgery was done. At the time of surgery we found a hemorrhagic cyst in left ovary, in the presence of normal looking fallopian tubes. In this case surgical team was suspecting ovarian ectopic pregnancy as the first possibility since all four criteria (Spiegelberh) was verified. But we were not 100% confident because the mass could be simple hemorrhagic cyst, and there was possibility of abortion of intrauterine pregnancy. So we underwent dilation & curettage just after laparoscopic surgery. This case demonstrates the importance of confirming by pathological reports and following beta-HCG concentrations postoperatively (until negative).
We also compared average gestational week, side of the lesion, average beta HCG levels between ovarian pregnancy group and tubal pregnancy group. In both groups, average gestational weeks (ovarian pregnancy, 7.1 vs. tubal pregnancy, 8.0 weeks), age (ovarian pregnancy, 33 vs. tubal pregnancy, 32.5 years) was not significantly different. From our study the number of total cases were not large enough to find out whether it is statiscally significant, but total beta HCG level showed difference remarkably between the two groups (ovarian pregnancy, 1,195.49 mIU/mL vs. tubal pregnancy, 11,579.55 mIU/mL). We found out that the beta HCG level was lower remarkably in ovarian pregnancy group. In ovarian pregnancy the lesion is on the surface of ovarian tissue which may be more vulnerable to the rupture and can lead to early rupture of the tissue. This may be the reason for the lower beta HCG level in ovarian pregnancy group, and further study may be needed to find out the correlation between beta HCG level and ovarian pregnancy and it may be helpful to make early diagnosis. Early diagnosis is fundamental to avoid more serious complications and an emergency invasive procedure.
Figures and Tables
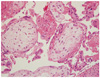
Fig. 2
Histopathology pictures of the specimen taken from the ovarian mass, showing chorionic vill which is the evidence of the product of conception (H&E, ×200).
References
1. Grimes HG, Nosal RA, Gallagher JC. Ovarian pregnancy: a series of 24 cases. Obstet Gynecol. 1983. 61:174–180.
2. Spiegelberg O. Casuistry in ovarian pregnancy. Arch Gynaekol. 1878. 13:73–79.
3. Raziel A, Golan A, Pansky M, Ron-El R, Bukovsky I, Caspi E. Ovarian pregnancy: a report of twenty cases in one institution. Am J Obstet Gynecol. 1990. 163:1182–1185.
4. al-Meshari AA, Chowdhury N, Adelusi B. Ovarian pregnancy. Int J Gynaecol Obstet. 1993. 41:269–272.
5. Tinelli A, Hudelist G, Malvasi A, Tinelli R. Laparoscopic management of ovarian pregnancy. JSLS. 2008. 12:169–172.
6. Chen JS, Ke TY, Hsieh PC. Heterotopic ovarian pregnancy. Taiwan J Obstet Gynecol. 2009. 48:193–195.
7. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998. 351:1115–1120.
8. Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999. 341:1974–1978.
9. Potter MB, Lepine LA, Jamieson DJ. Predictors of success with methotrexate treatment of tubal ectopic pregnancy at Grady Memorial Hospital. Am J Obstet Gynecol. 2003. 188:1192–1194.
10. Takacs P, Rodriguez L. High folic acid levels and failure of single-dose methotrexate treatment in ectopic pregnancy. Int J Gynaecol Obstet. 2005. 89:301–302.
11. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing "single dose" and "multidose" regimens. Obstet Gynecol. 2003. 101:778–784.
12. Takacs P, Chakhtoura N, De Santis T, Verma U. Evaluation of the relationship between endometrial thickness and failure of single-dose methotrexate in ectopic pregnancy. Arch Gynecol Obstet. 2005. 272:269–272.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download