Abstract
Given the extended survival of patients diagnosed with cervical cancer, a large number of women are treated with radiotherapy. A second primary cancer has currently become one of the most important radiation-induced injuries as the number of cancer patients who are cured is increasing by virtue of recent advancements in therapeutic radiology. A 56-year-old woman developed two pelvic malignancies 14 years after undergoing surgery and radiotherapy for adenosquamous cell carcinoma of the cervix. Previous exposure to radiotherapy is associated with certain malignancies, and review of the literature indicates that there is strong evidence to support such an association with bladder and ovarian cancer. This is the first reported case of second multiple primary malignancies (ovarian cancer and bladder cancer) after radiation therapy for adenosquamous cell carcinoma of the cervix.
The incidence of multiple primary malignancies (MPMs) has increased in recent decades. This increase in the diagnostic rate of MPMs may be because of patients surviving long enough to develop another primary cancer, due to the involvement of common or different intrinsic/extrinsic factors or carcinogens, such as genetic predisposition resulting in the cancer family syndrome, DNA-damaging toxins, exposure to carcinogens (for example, smoking), hormones, dietary factors, previous radiotherapy and chemotherapy [1].
Radiotherapy (RT) is widely used to manage invasive uterine cervical cancer, and more than 65% of patients with invasive cancer are treated with this therapy worldwide [2]. RT consists of a combination of high-dose-rate intracavitary brachytherapy (ICBT) and external beam radiotherapy (EBRT) [3]. RT for cervical cancer is known to cause various malignancies, including cancer of the bladder, rectum, vagina, and cecum. Second primary cancers are defined as those differing histologically from the primary cancer, should arise in different situations, and must be seen to produce their own separate metastases [4]. Triple primary malignant cancers of the female pelvic organ, however, are uncommon. We review a case of a woman who presented with MPMs (ovarian cancer and bladder cancer) after receiving radiotherapy for adenosquamous cell carcinoma of the cervix.
A 58-year-old woman (gravida 4, para 4), visited our hospital for evaluation of a pelvic mass and hematuria, the onset of which was one month ago. Fourteen years ago, she received a transabdomial hysterectomy (TAH) with right salpingo-oophorectomy due to uterine myoma and severe dysplasia of the cervix, which was diagnosed by colposcopy guided punch biopsy. But the postoperative pathologic diagnosis was confirmed as adenosquamous cell carcinoma of the uterine cervix (International Federation of Obstetrics and Gynecology stage Ib) (Fig. 1), so the patient was treated with radiotherapy: external radiation (50.4 Gy) in 28 fractions and high-dose rate (HDR) ICBT (20 Gy) in 7 fractions over 2 months.
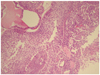
Recently, the patient had feelings of abdominal distension, low abdominal discomfort and hematuria. She was a non-smoker, and her family history of cancer was non-specific. Her vital signs were unremarkable, and on physical examination, there was abdominal distention with slight tenderness. An immobile, palpable abdominal mass which had an approximate size of a large fist was discovered, and was thought to be an omental cake mass or ovarian mass. Multiple papillary masses were found at trigone, right lateral and posterior wall of bladder by cystoscope. Transvaginal and transabdominal sonographies showed a large amount of ascites in the peritoneal cavity and perihepatic area, as well as a 6×5 cm-sized cystic pelvic mass at the left adnexal site. The differential diagnosis included ovarian cancer and metastatic cancer. We performed an abdomino-pelvic computed tomography (CT) with intravenous administration of a non-ionic iodinated contrast agent. The CT showed irregular nodular peritoneal thickening, an omental cake at the subhepatic and lower pelvic cavities, and a lowdensity elongated multiloculated cystic mass, about 5.9×2.2×4.9 cm in size, in the pelvic cavity (Fig. 2). It was suspected to be serous papillary adneocarcinoma with extensive peritoneal carcinomatosis in the left adnexa with huge amounts of ascites in the whole abdominal cavity. Laboratory tests showed no abnormalities in hematologic and biochemical data. Serum levels of the tumor markers cancer antigen (CA) 125, CA 19-9 and squamous cell carcinoma related antigen were 2,224.6 U/mL (normal range, 2.4-36.3 U/mL), 6.9 ngU/mL (normal range, 0-37 U/mL), 1.75 ng/mL (normal range, 0-1.5 ng/mL) and ascitic fluid level of carcinoembryogenic antigen was 0.8 U/mL, respectively. The CA 125 level was very high and the postoperative serum CA 125 level was 111 U/mL. Ascites fluid cytology was positive for malignant cells, making it most likely metastatic adenocarcinoma. On the basis of these results, the abdominal mass was diagnosed as an ovarian cancer with abdominal metastasis. One week later she underwent exploratory laparotomy. At the time of surgery, gross findings included the following: huge ascites, multiple metastasis at the peritoneal wall, omemtum, distal colon, rectum, bladder wall (multiple papillary mass in trigon, posterior and right lateral wall), and a cystic and solid left ovary with an irregular margin. Therefore, we performed a left salpingo-oophorectomy, adhesiolysis, total omentectomy, debulking operation, and transurethral resection of the bladder tumor with biopsy. The histopathologic findings revealed multiple metastases of an ovarian cancer, a serous carcinoma (grade 3) of an ovary (Fig. 3) and papillary transitional cell carcinoma (low-grade) in the urinary bladder (Fig. 4). Immunohistochemical studies showed low proliferative activity of Ki-67, positive CK7 and focally positive CK20 in bladder cancer (Fig. 5). There were no significant complications during the post-operative hospital stay while the patient received chemotherapy.
MPMs in a single patient were first reported by Billroth [4] in 1889. Many cases of MPMs of the female genital tract have been described since then. In 1961, Werthamer et al. [5] in describing a case of quadruple primary malignancy, put forward more detailed criteria. He suggested that 1) There must be histological evidence of primary tumors, 2) Paired organ malignancies whether synchronous or metachronous, must be considered as one tumor, 3) Multiple tumors in the same organ must be considered as a single primary tumor, 4) The lower intestinal tract as well as the uterus are considered as a single organ, 5) A careful histological attempt to exclude metastases should be made. MPMs may be synchronous or metachronous depending on the interval between their diagnosis. Synchronous cancers are diagnosed simultaneously or within an interval of about 6 months, and metachronous cancers are secondary cancers that developed more than 6 months after the diagnosis of primary cancers usually after treatment of the primary lesions [6].
MPMs were classified into four types: 1) Multicentric, if the two distinct carcinomata arise in the same organ or tissue, 2) systemic, if they arise on anatomically or functionally allied organs of the same system (colon and rectum cancers), 3) Paired organs, as in the breasts, and 4) Random, if they occur as a co-incidental or accidental association in unrelated sites [7].
The most frequent synchronous primary neoplasms of the female genital lesions were ovarian and endometrial cancer. Takeda et al. [1] reported a retrospective analysis of 1,044 patients with gynecologic malignancies treated over a 12-year period, in order to review the frequency and types of MPMs. MPMs were detected in 45 (4.3%) cases, including 16 (2.1%) out of 733 cervical cancers, 14 (8.2%) out of 166 endometrial cancers, 3 (15%) out of 20 vaginal cancers, and 12 (9.8%) out of 123 ovarian cancers. Fifteen cases were synchronous and the remaining 24 cases were heterochronous, with an average 4.9-year interval. The next most frequent site of neoplasm was the breast, particularly in patients with endometrial or ovarian cancer. They concluded that gynecologic malignancies are often associated with primary cancers elsewhere, especially in the breast, stomach, colon and thyroid.
Other urogenital organs such as the uterus, ovaries and bladder receive large doses of radiation during treatment of cervical cancer, and thus it is not surprising that the risk for cancer at these organs increases with the duration of time since the exposure, and that there is a significant excess of cancer of "other urogenital organs."
Definitive RT consists of a combination of EBRT for the pelvis and ICBT, using HDR or low-dose rate sources such as radium, or cesium, or a combination of both modalities. Radiotherapy for cervical cancer resulted in very high doses of radiation (>30 Gy) to organs in the pelvic region, such as the ovaries, rectum, and bladder, whereas organs in the abdominal cavity, such as the stomach and pancreas, received between 1 and 3 Gy [8].
Occurrence of a second primary cancer is a most serious event among long-term survivors after RT. Boice et al. [9] examined data from 15 cancer registries in eight countries and compared the number of second primary cancers reported for 182,040 women against the number expected to have the same risk prevalent in the general population. They found an increased risk for cancers of the bladder, rectum, vagina, and cecum [10]. In another study, Arai et al. [11] reported significantly higher incidences of second primary cancers in the rectum, bladder, and lung as well as leukemia. Kleinerman et al. [12] described a large-scale study of 49,828 patients with cervical cancer treated with radiation therapy, 3,750 survived 30 or more years and a two-fold risk of cancers of heavily irradiated organs was seen in 16,713 matched patients treated without radiotherapy. They reported that most of the second primary cancers were of the rectum, vagina, vulva, and bladder, and they concluded that radiation is an important cause of the second primary cancers, with no evidence that the risk returns to a normal level. There have been a few studies in which both relative risk (RR) and cumulative risk (CR) beyond 20 years after RT were quantified in patients with cervical cancer. They also reported that RR per decade for second primary cancers after cervical cancer treated with RT was 1.2 for 1 to 9 years, 1.2 for 10 to 19 years, 1.3 for 20 to 29 years, and 1.6 after 30 years, suggesting a continuing elevation of RR of second primary cancers with longer follow-ups. They also reported that RR in heavily or moderately irradiated organ sites among 10-year survivors was 2.1 for patients aged <40 years, 1.6 for those 40 to 49 years, 1.4 for those 50 to 59 years, and 1.2 for patients >60 years, indicating an inverse correlation with age of RT. In Japan, Ohno et al. [13] described a study of 2,167 patients with cervical cancer treated with radiation therapy; the total number of person-years of follow-up was 25,771. Among the 2,167 patients, 1,063 (49%) survived more than 10 years. Second primary cancers were observed in 210 patients, representing a significant 1.2-fold risk of developing second primary cancer compared with the general population, 1.6% excess risk per person per decade of follow-up, and elevating CR up to 23.8% at 30 years after radiotherapy. As is well known, smoking is strongly associated with cancer of the lung, head and neck, esophagus, urinary bladder, pancreas, stomach, liver, kidney, cervix, and myeloid leukemia. They also reported that the impact of smoking habits on the increasing risk of second primary cancer after cervical cancer treated with RT. In their study, the RR of second primary cancer was 1.6-fold for patients with a smoking history but 1.4-fold for patients without such a history, revealing a relatively higher RR in the former.
In this case, our patient had TAH and radiotherapy for adenosquamous cell cancer of the uterine cervix, after which she developed ovarian and bladder cancer fourteen years later. Although the incidence of MPMs after radiotherapy is low, careful vigilance should be exercised during the follow-up period. The early detection of second primary malignancies will enable prompt management and will increase the cure rate of the disease.
Figures and Tables
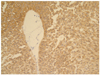
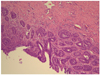 | Fig. 1Low-magnified view of an adenosquamous carcinoma show a glandular differentiation with multilayered growth. It also reveals a squamous differentiation in a big tumor cell cluster with sheet-like and focally whorling apperances (H&E, ×100). |
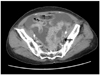 | Fig. 2The axial view of post-contrast computed tomography showed a pelvic mass with a huge ascites. The margin is irregular and cystic-solid mass in pelvic cavity (arrow). |
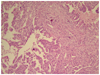 | Fig. 3Histologically, the tumor of ovary consists of polygonal pleomorphic cells arranged in solid sheets or lacy pattern. Psammoma bodies are also present (H&E, ×100). |
References
1. Takeda T, Sagae S, Koizumi M, Terasawa K, Ishioka S, Takashima S, et al. Multiple primary malignancies in patients with gynecologic cancer. Int J Gynecol Cancer. 1995. 5:34–39.
2. Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, et al. Carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2003. 83:Suppl 1. 41–78.
3. Barillot I, Horiot JC, Pigneux J, Schraub S, Pourquier H, Daly N, et al. Carcinoma of the intact uterine cervix treated with radiotherapy alone: a French cooperative study: update and multivariate analysis of prognostics factors. Int J Radiat Oncol Biol Phys. 1997. 38:969–978.
4. Billroth T. Die allgemeine chirurgische pathologie und therapie. 1889. Berlin: G. Reimer.
5. Werthamer S, Jabush M, Schulman J. Multiple primary malignancies. JAMA. 1961. 175:558–562.
6. Matlock DL, Salem FA, Charles EH, Savage EW. Synchronous multiple primary neoplasms of the upper female genital tract. Gynecol Oncol. 1982. 13:271–277.
7. Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. II. Tumors of different tissues or organs. Cancer. 1961. 14:231–237.
8. Stovall M, Smith SA, Rosenstein M. Tissue doses from radiotherapy of cancer of the uterine cervix. Med Phys. 1989. 16:726–733.
9. Boice JD Jr, Engholm G, Kleinerman RA, Blettner M, Stovall M, Lisco H, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988. 116:3–55.
10. Boice JD Jr, Day NE, Andersen A, Brinton LA, Brown R, Choi NW, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst. 1985. 74:955–975.
11. Arai T, Nakano T, Fukuhisa K, Kasamatsu T, Tsunematsu R, Masubuchi K, et al. Second cancer after radiation therapy for cancer of the uterine cervix. Cancer. 1991. 67:398–405.
12. Kleinerman RA, Boice JD Jr, Storm HH, Sparen P, Andersen A, Pukkala E, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995. 76:442–452.
13. Ohno T, Kato S, Sato S, Fukuhisa K, Nakano T, Tsujii H, et al. Long-term survival and risk of second cancers after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2007. 69:740–745.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download