Abstract
Struma ovarii is defined as a rare monodermal form of ovarian teratoma that occupies more than 50% of thyroid tissue. Only 2% of struma ovarii display malignant histology, most of which is revealed as papillary carcinoma type. Follicular carcinoma originating from malignant struma ovarii is rarely reported. We report a case of a pregnant woman diagnosed with peritoneal dissemination of follicular carcinoma arising from struma ovarii.
The frequency of histological detection of thyroid tissue in ovarian mature teratoma is less than 20%, while that of a macroscopic detection is less than 3% [1]. Struma ovarii is defined as a rare monodermal form of ovarian teratoma, which comprises of more than 50% of thyroid tissue [2]. Mature cystic teratoma is a benign tumor, 2% of which have been reported to be struma ovarii [3]. Histologic malignancy arising from struma ovarii has been rarely reported and its occurrence is less than 5% in struma ovarii. Most histologic malignancies arising from struma ovarii are papillary thyroid carcinoma, and account for 70%−85% of total cases [3,4]. Follicular carcinoma of histologic malignancy arising from struma ovarii is extremely rare. Here, an early pregnant woman with follicular carcinoma arising from struma ovarii is reported with a review of relevant literature.
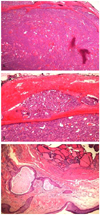
A 33-year-old, pregnant woman with gravida 3, para 1 was referred under the impression of bilateral ovarian malignancy, which was detected incidentally via a routine sonography in early pregnancy. The patient had two surgical histories: laparoscopic right ovarian cystectomy of mature cystic teratoma, five years ago, and laparoscopic cholecystectomy of gall bladder stone, two years ago. Pelvic magnetic resonance imaging (MRI) revealed bilateral parovarian solid tumors near the pelvic sidewall and unusual manifestation of metastatic lesions in the pelvic cavity (Fig. 1). On March 2, 2010, the patient underwent explolaparotomy under the impression of bilateral ovarian malignancy at 7+6 weeks of intrauterine pregnancy. The patient had a slightly enlarged uterus appropriate for eight weeks gestational age, and the bilateral ovary and fallopian tube were normal in size and shape. About ten metastatic masses with pink color and rubbery consistency were observed on the peritoneum and the surface of the colon. The greatest mass was noted on the abdominal peritoneum under the umbilicus, and it was 5 × 4 cm in size. The cut surface showed homogeneously pink color with soft contour. Frozen histologic diagnosis revealed it to be a struma ovarii. On final histopathologic findings, the peritoneal masses were found to have a closed-packed small- and medium-sized thyroid gland enveloped in a fibrous capsule (Fig. 2A). Follicular carcinoma featured remnants of vascular invasion, in the blood vessels outside of the fibrous capsule (Fig. 2B). Fig. 2C illustrates the previous mature cyst teratoma which was removed at another hospital 5 years ago. It was composed of mature tissue resembling epidermis tissue. In some areas, there were thyroid follicles accounting for less than 10% of the mature cystic teratoma. On the postoperative thyroid evaluation, sonography of thyroid and thyroid function test were carried out. The ultrasound scan of the thyroid gland was normal and the serum T3 and free T4 levels were 123.3 ng/dL (normal range, 71−161 ng/dL) and 1.6 ng/dL (normal range, 0.8−1.7 ng/dL), respectively. The thyroid stimulation hormone level was 0.10 uIU/mL (normal range, 0.86−4.69 uIU/mL).
The patient delivered a healthy male baby on intrauterine pregnancy at 40th weeks and underwent total thyroidectomy and radioactive iodine therapy.
The most commonly reported malignancy of struma ovarii is papillary carcinoma. In contrast, follicular carcinoma has been reported to occur in an extremely small number of malignant cases of struma ovarii, as thus only few cases have yet been disclosed.
Histologic diagnosis of follicular carcinoma arising from struma ovarii is more difficult than that of a cervical thyroid cancer. Capsular invasion is a key factor for making a diagnosis. In comparison to cervical thyroid, the capsule enveloping the follicular carcinoma arising from struma ovarii is not frequently found. In these cases, a diagnosis of follicular carcinoma originating from struma ovarii is made based on the presence of vascular invasion, an invasion to the ovarian tissue enveloping the follicular carcinoma and metastasis [5].
Thyroid-type carcinoma arising from struma ovarii can metastasize to the peritoneum and lymph node following an invasion to ovarian serosa and further metastasizes to organs such as liver, lung, bone and vaginal vault [6-8]. In the cases of histologic malignancy, however, a clinically favorable course has been reported frequently despite dissemination to other sites. Even in cases in which a malignancy was present on histology, there was a good prognosis with a 10-year survival of 81% and a 25-year one of 60% from malignant struma ovarii of all cell types [4]. In summary, malignant histology of struma ovarii does not always coincide with the malignant clinical course.
In our case, resection of mature cystic teratoma was performed 5 years ago. At that time, a histology review showed that thyroid tissue accounted for less than 10% of the mature cyst teratoma. However, a small portion of thyroid tissue was implanted in the abdominal and peritoneal cavity during the laparoscopic surgery. Five years later, the late recurrence and peritoneal dissemination were observed. In particular, the main mass was found at the trocar site under the umbilicus.
Thyroid-type carcinoma, including follicular cell-type, arising from struma ovarii frequently shows gradual progression without clinical symptom. In our case, the tumor was detected via a routine sonography during first trimester. There have also been some cases in which late recurrence occurred. Roth and Karseladze [9] reported a case of peritoneal dissemination, observed 26 years after the removal of a struma ovarii.
In cases of cervical thyroid cancer, it is generally known that papillary carcinoma has a relatively better prognosis as compared to that of follicular carcinoma. It is also expected that follicular carcinoma arising from struma ovarii will have a similar tendency toward developing cervical thyroid cancer. According to Roth et al. [10], follicular carcinoma originating from struma ovarii had a 45% dissemination frequency, which was higher than the 19% seen in cases of papillary carcinoma. These authors also noted that the incidence of bone metastasis was 19%, which was also higher than the 4% seen in cases of papillary carcinoma.
To date, there are no established treatment guidelines for adjuvant therapy of malignancy originating from struma ovarii. Yet, total thyroidectomy and radioactive iodine therapy are strongly recommended. The reason is that a differential diagnosis from the primary origin should be made because there are rare cases of cervical thyroid cancer in which an abdominal metastasis is present and the effectiveness of radioactive iodine therapy need to be enhanced. In addition, radioactive iodine can confirm and treat residual thyroid-type carcinoma. Furthermore, recurrence can be confirmed at follow-up using serum thyroglobulin level and radioactive iodine [8,11]. To date, however, total thyroidectomy and radioactive therapy have not been accepted as a standard treatment regimen. This is because a malignancy originating from struma ovarii progresses slowly and it also exhibited good long-term survival rate even in patients who did not undergo total thyroidectomy [12,13]. Long-term survival was also observed in patients who had a recurrence.
In the current case, thyroid evaluation was performed after histopathological diagnosis. Thyroid ultrasonography and serum thyroid hormone levels showed normal findings. The patient delivered a healthy male baby on intrauterine pregnancy at 40th weeks and underwent total thyroidectomy followed by radioactive iodine therapy. In cases of malignant struma ovarii including follicular carcinoma, local resection should be performed, and adjuvant therapy may be selected on a case-by-case basis.
Figures and Tables
Fig. 1
Pelvic magnetic resonance imaging reveals (A) 4.5 × 3.0 cm sized right parovarian solid tumor, (B) an intrauterine gestational sac containing a fetal pole and 2.5 × 2.5 cm sized left parovarian solid tumor and 1.5 × 1.0 cm sized metastatic lesion in the pelvic cavity.

Fig. 2
Follicular thyroid type carcinoma arising in struma ovarii. (A) Peritoneal mass: tumor is enveloped by fibrous capsule and composed of closely packed small- and medium-sized follicles of thyroid gland (H&E, ×12). (B) Follicular carcinoma demonstrating vascular invasion. Tumor plug is present within the blood vessel located outside the fibrous capsule (H&E, ×100). (C) Mature cystic teratoma resected 5 years ago. Lesion consists of mature tissue resembling epidermis in lower left side and macro- and microfollicles of thyroid tissue in upper right side (H&E, ×40).
Acknowledgments
This work was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (NRF-2008-314-E00121), 311-2006-2-E00339 and 2010-0011153; by a faculty research grant from the Yonsei University College of Medicine for 2010 (6-2010-0110); and a grant funded by Sanofi-Aventis Korea and CJ.
References
1. Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament: atlas of tumor pathology. 1998. Washington, DC: Armed Forces Institute of Pathology.
2. Celestino R, Magalhães J, Castro P, Triller M, Vinagre J, Soares P, et al. A follicular variant of papillary thyroid carcinoma in struma ovarii. Case report with unique molecular alterations. Histopathology. 2009. 55:482–487.
3. Yücesoy G, Cakiroglu Y, Muezzinoglu B, Besnili B, Yucesoy I. Malignant struma ovarii: a case report. J Korean Med Sci. 2010. 25:327–329.
4. Robboy SJ, Shaco-Levy R, Peng RY, Snyder MJ, Donahue J, Bentley RC, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol. 2009. 28:405–422.
5. Roth LM, Talerman A. The enigma of struma ovarii. Pathology. 2007. 39:139–146.
6. Chan SW, Farrell KE. Metastatic thyroid carcinoma in the presence of struma ovarii. Med J Aust. 2001. 175:373–374.
7. McDougall IR. Metastatic struma ovarii: the burden of truth. Clin Nucl Med. 2006. 31:321–324.
8. Zekri JM, Manifold IH, Wadsley JC. Metastatic struma ovarii: late presentation, unusual features and multiple radioactive iodine treatments. Clin Oncol (R Coll Radiol). 2006. 18:768–772.
9. Roth LM, Karseladze AI. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol. 2008. 27:213–222.
10. Roth LM, Miller AW 3rd, Talerman A. Typical thyroid-type carcinoma arising in struma ovarii: a report of 4 cases and review of the literature. Int J Gynecol Pathol. 2008. 27:496–506.
11. Volpi E, Ferrero A, Nasi PG, Sismondi P. Malignant struma ovarii: a case report of laparoscopic management. Gynecol Oncol. 2003. 90:191–194.
12. O'Connell ME, Fisher C, Harmer CL. Malignant struma ovarii: presentation and management. Br J Radiol. 1990. 63:360–363.
13. Karseladze AI, Kulinitch SI. Peritoneal strumosis. Pathol Res Pract. 1994. 190:1082–1085.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download