Abstract
Objective
To examine the relationship between amniotic fluid (AF) white blood cell (WBC) count and the presence and severity of inflammatory lesions of the placenta in women with preterm premature rupture of membranes (PPROM).
Methods
This retrospective cohort study included 90 consecutive women with PPROM (24.0-35.6 weeks) who met the following criteria: singleton gestation; transabdominal amniocentesis performed to obtain AF for culture and WBC count; delivery within 72 hours of amniocentesis; placental histologic examination after preterm delivery.
Results
The prevalence of histologic chorioamnionitis was 32% (29/90) and that of positive amniotic fluid culture was 21% (19/90). Patients with histologic chorioamnionitis had a significantly higher AF WBC count than those without this lesion. Logistic regression analysis demonstrated that AF WBC count had a significant relationship with histologic chorioamnionitis after controlling for gestational age and AF culture. The median AF WBC count increased significantly according to the higher severity of inflammation in each type of placental histologic section. According to receiver operating characteristic curve analysis, the best cutoff value of AF WBC count for predicting histological chorioamnionitis was 25 cells/mm3, with a sensitivity of 62% and a specificity of 77%.
REFERENCES
1. Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol. 1991; 165:1105–10.

2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008; 371:75–84.

3. Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993; 169:839–51.

4. Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996; 88:1034–40.
5. Zlatnik FJ, Gellhaus TM, Benda JA, Koontz FP, Burmeister LF. Histologic chorioamnionitis, microbial infection, and prematurity. Obstet Gynecol. 1990; 76:355–9.

6. Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992; 166:1382–8.

7. Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010; 116:387–92.
8. Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. Canadian Neonatal Network. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009; 200:372.e1–6.

9. Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Neonatal Med. 2009; 22:780–4.

10. Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996; 97:210–5.

11. Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999; 181:773–9.

12. Kim JC, Yoon BH. The relationship between amniotic fluid white blood cell count and the presence and severity of acute placental inflammation in preterm premature rupture of membrane. Korean J Obstet Gynecol. 2000; 43:885–90.
13. Kim M, Yoon BH. The diagnostic and prognostic value of amniotic fluid white blood cell count in patients with preterm premature rupture of the membranes. Korean J Obstet Gynecol. 2002; 45:101–11.
14. Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991; 165:821–30.

15. Steel JH, O'Donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta. 2005; 26:672–7.

16. Park KH, Yoon BH, Choe G, Jun JK, Syn HC. Prenat Neonatal Med. The relationship between the presence, severity, and pattern of acute placental inflammation and amniotic fluid white blood cell count in preterm labor. 1997; 2:294–9.
17. Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993; 169:805–16.

18. Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990; 163:968–74.

19. Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995; 172:960–70.

20. Greig PC, Ernest JM, Teot L, Erikson M, Talley R. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol. 1993; 169:1035–44.

21. Park KH, Yoon BH, Kim MH, Kim GJ, Kim T, Lee HK, et al. A comparative study of the diagnostic value of amniotic fluid interleukin-6 and culture for the antenatal diagnosis of intrauterine infection and prediction of perinatal morbidity in patients with preterm premature rupture of membranes. Korean J Obstet Gynecol. 2000; 43:1019–28.
22. Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intraamniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004; 191:1339–45.

23. Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002; 8:3–13.
Fig. 1.
Receiver operating characteristic curve for amniotic fluid white blood cell in predicting the occurrence of histologic chorioamnionitis. Number next to solid dots represents cutoff value of white blood cell (area under the curve 0.716; standard errors 0.065; P=0.001).
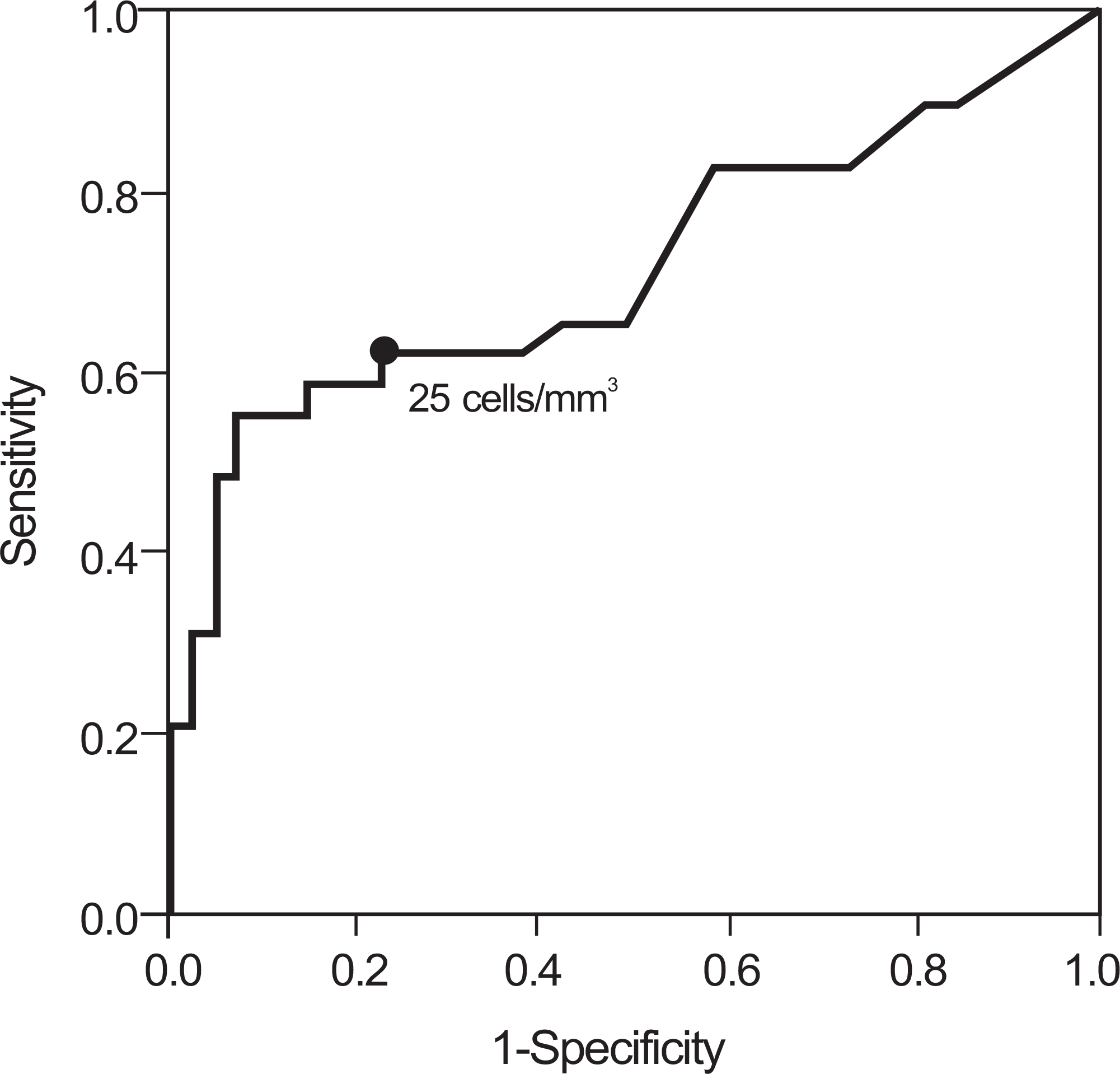
Table 1.
Histological grade for acute intrauterine inflammation
Table 2.
Demographic and clinical characteristics of the study population according to the histologic chorioamnionitis
Table 3.
Risk factors associated with histologic chorioamnionitis after adjustment of confounding variables by logistic regression
Table 4.
Amniotic fluid culture and white blood cell count according to the presence and severity of inflammation in each type of placental histologic section
| Tissue | n | Positive amniotic fluid culture | P‐value∗ | Amniotic fluid WBC | P‐value† |
|---|---|---|---|---|---|
| Amnion | |||||
| Grade 0 | 82 | 14 (17) | 0.006 | 6.5 (0‐10880) | 0.067 |
| Grade 1 | 6 | 3 (50) | 910 (0‐8160) | ||
| Grade 2 | 1 | 1 (100) | 12100 | ||
| Chorion‐decidua | |||||
| Grade 0 | 62 | 5 (8) | <0.001 | 5 (0‐2020) | 0.001 |
| Grade 1 | 18 | 9 (50) | 55 (0‐8160) | ||
| Grade 2 | 10 | 5 (50) | 2120 (0‐12100) | ||
| Umbilical cord | |||||
| Grade 0 | 77 | 11 (14) | <0.001 | 5 (0‐12100) | <0.001 |
| Grade 1 | 6 | 3 (50) | 975 (5‐5440) | ||
| Grade 2 | 7 | 5 (71) | 1600 (80‐10880) | ||
| Chorionic plate | |||||
| Grade 0 | 78 | 14 (18) | 0.021 | 6 (0‐9760) | 0.001 |
| Grade 1 | 6 | 2 (33) | 17.5 (0‐1680) | ||
| Grade 2 | 5 | 3 (60) | 8160 (1001‐12100) |
Table 5.
Amniotic fluid white blood cell count according to the total grade of histologic chorioamnionitis
| Total grade of histologic chorioamnionitis | n | Amniotic fluid white blood cell | P‐value∗ |
|---|---|---|---|
| Grade 0 | 61 | 5 (0‐2020) | <0.001 |
| Grade 1 or 2 | 14 | 5 (0‐1880) | |
| Grade 3 or 4 | 9 | 680 (0‐9760) | |
| Grade 5 or 6 | 6 | 4920 (1001‐12100) | |
| Grade 7 or 8 | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download