Abstract
Spontaneous rupture of mature cystic teratoma occurs rarely, but may lead to a chemical peritonitis. Once rupture of mature cystic teratoma is diagnosed, immediate surgical intervention is necessary. Removal of ruptured ovarian cystic teratoma and copious lavage of abdominal cavity are usually sufficient to prevent prolonged chemical peritonitis. We report here a rare case of spontaneously ruptured ovarian cystic teratoma diagnosed by computed tomography scan obtained before and after the rupture, and in which chemical peritonitis lasted over 2 months after surgery.
Mature cystic teratomas are the most common ovarian tumors, accounting for 20% of adult ovarian tumors and 50% of pediatric ovarian tumors. The most frequent complication of an ovarian mature cystic teratoma is torsion, but rupture occurs rarely with an estimated incidence of 0.3-2.5% [1,2]. Its spontaneous or iatrogenic intraperitoneal rupture may lead to a chemical peritonitis. We report a case of intraperitoneal rupture of an ovarian mature cystic teratoma, diagnosed by repeated computed tomography (CT) at short intervals, and resultant chemical peritonitis which persisted for over 2 months after surgery.
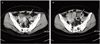
A 50-year-old woman, gravida 3, para 2, presented to our emergency room with several hours history of abdominal pain and nausea. On physical examination, she was afebrile and vital signs were stable. Abdominal examination revealed mild tenderness in lower abdomen. No obvious muscle guarding or rebound tenderness was noted. Total white blood cell count was 12,980/mm3 and C-reactive protein (CRP) was 12.9 mg/dL. Abdominal CT scan showed cystic mass measuring 4.2 cm in maximal diameter with fat-fluid level in left adnexa, suggestive of a cystic teratoma, and small amount of ascites in pelvic cavity (Fig. 1A). No other abnormalities suspicious of torsion or rupture of a cystic teratoma were found. Diagnostic laparoscopy had been recommended for further evaluation, but she signed herself out of the hospital against medical advice for personal reasons. Two days later, she readmitted to the emergency room with progressively worsening abdominal pain. Physical examination revealed a distended abdomen with marked tenderness and rebound tenderness in the lower abdomen. The body temperature was elevated to 38.8℃. The serum CRP rose to 33.4 mg/dL. Serum CA 19-9 was 75.6 U/mL (normal value 0-27 U/mL) and CA-125 was 39.7 U/mL (normal value 0-35 U/mL). A repeated CT scan of the abdomen and pelvis demonstrated a left ovarian cystic teratoma measuring 3 cm in maximal diameter surrounded by fat globules (Fig. 1B). The size of an ovarian cyst and the amount of fat component within the cyst were decreased compared with previous CT scan. Thickening of peritoneum, diffuse wall thickening of small bowels, and increased amount of ascites were also noted. Based on CT findings as well as the clinical symptoms, a diagnosis of ruptured ovarian cystic teratoma and resultant chemical peritonitis was made.
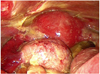
Single-port laparoscopic surgery using OCTO Port (Dalim SurgNet, Seoul, Korea) was performed. Operative findings revealed about 1,500 mL of yellowish ascites containing adipose component. The peritoneum, uterus, and bowel surfaces were covered by diffuse, thick, white to yellowish plaque-like lesion (Fig. 2). Multiple flimsy adhesions were present between the omentum and bowel loops. The left ovarian cyst was adherent to the pelvic sidewall. Left oophorectomy was performed and meticulous peritoneal irrigation with 18 L of warm saline was carried out until no more particles of fatty tissue could be detected in the solution. Pathologic examination confirmed the diagnosis of mature cystic teratoma. The ascitic fluid cytology was negative for malignant cells. During the perioperative period, she received intravenous cefotaxime 2 g once daily and metronidazole 500 mg every 8 hours.
After an uneventful immediate postoperative course, the patient complained of abdominal pain, fever, nausea, and diarrhea from the postoperative day 4. With a diagnosis of antibiotic-induced pseudomembranous colitis, oral metronidazole was started. However, stool enzyme immunoassays for Clostridium difficile toxins were negative and pseudomembranes were not visualized by colonoscopy. Blood cultures for aerobic and anaerobic bacteria did not produce any growth and stool culture demonstrated only normal flora. A CT scan revealed large amount of ascites in abdominal cavity. Ascites were drained by percutaneous placement of a pigtail catheter and sent for analysis. Examination of the ascitic fluid showed a white blood cell count of 1,600/µL (52% lymphocytes and 25% polymorphonuclear leukocytes) and was negative for acid-fast bacilli and gram stain. A culture of the fluid for bacteria was sterile. The adenosine deaminase level was 30 IU/L and polymerase chain reaction for M. tuberculosis was negative. The fever over 38oC continued and the severity of abdominal pain, nausea, vomiting, and diarrhea increased. The CRP level, which had been gradually decreased to 12.5 mg/dL on postoperative day 7, increased to 19.7 mg/dL on postoperative day 9.
Persistent chemical peritonitis was suspected and, following extensive discussion and journal search, prednisolone at a dose of 40 mg orally daily was initiated from postoperative day 9. After administration of prednisolone, all of her symptoms gradually improved. She was discharged on postoperative day 16. However, 5 weeks later when the dosage of prednisolone had been tapered to 10 mg per day, the symptoms and signs of peritonitis redeveloped. Therefore, prednisolone 15 mg per day was administered for another one month and then tapered off. CRP level was normalized. At present, one year after the surgery, the patient is under follow-up at the out-patient clinic, and is doing well, with no symptoms or signs of chemical peritonitis.
Mature cystic teratoma of the ovary is the most common ovarian neoplasm, accounting for approximately 5-25% of all ovarian tumors [2]. It presents most commonly during the reproductive years and is bilateral in 8-15% of cases. Most of the patients with teratoma are asymptomatic, and the adnexal mass is usually discovered on a routine ultrasonographic examination or with calcifications in the routine abdominal X-ray image. Complications of mature cystic teratoma of the ovary are torsion (16%), malignant degeneration (2%), rupture (1-2%), and infection (1%) [3].
Spontaneous rupture is an extremely rare complication of mature cystic teratoma because of its usually thick capsule. The exact cause of the rupture is mostly unknown, but torsion with infarction of the tumor, direct trauma or prolonged pressure from pregnancy or delivery, infection of the dermoid contents, malignant change, and internal pressure from rapid growth of the cyst are cited as plausible explanations [4,5]. Mature cystic teratoma may rupture into the peritoneal cavities or, less frequently, into the adjacent hollow viscus, such as the bladder, small bowel, rectum, sigmoid colon, vagina, and even through abdominal wall [3].
Leakage of sebaceous material from the intraperitoneal rupture of mature cystic teratoma causes an aseptic inflammatory peritoneal reaction (chemical peritonitis). The incidence of chemical peritonitis after rupture or leakage of cystic fluid in the peritoneum is less than 0.5% [2]. The clinical presentation may be divided into two categories; acute and chronic [4]. Acute peritonitis caused by sudden rupture of tumor contents may result in acute abdominal crisis and shock, and is usually associated with torsion, trauma, infection, labor, or physical exercise [3,6]. Chronic granulomatous peritonitis, which is more common than acute episode, results from a tiny perforation and slow leakage from a breach in the cyst wall [7]. The symptoms and signs might be subtle and marginal in the early period, however, the patient would complain of progressive abdominal distention, low abdominal pain, and gastrointestinal disturbances such as anorexia, nausea, vomiting, and diarrhea [4]. This is in good agreement with our case in which insidious leakage of contents into the peritoneal cavity, although not visualized on initial CT scan, might have caused mild peritonitis at first visit. Chronic granulomatous peritonitis is characterized by thick white to yellowish plaque-like lesion on the visceral peritoneum, especially on the surface of the uterus and rectum, dense adhesions, and variable ascites that simulate peritoneal carcinomatosis or tuberculous peritonitis [8].
An accurate diagnosis of a ruptured ovarian teratoma can be accomplished when the discontinuity of the wall is noted at ultrasonography (US), CT, and magnetic resonance (MR) imaging [9]. The presence of ascites and a distorted or flattened shape of the tumor suggest tumor rupture. The diagnosis of ruptured ovarian teratomas using CT imaging is fairly straightforward because this modality is very sensitive for detection of intraperitoneal fatty implant, most commonly around liver surface [3,9,10]. However, the early diagnosis of intraperitoneal rupture of ovarian teratoma as happened in this case is difficult. In the present case, we performed abdominal CT at short intervals and could detect apparent changes associated with tumor rupture, that is, decrease in tumor size, decreased amount of fat component within the cyst, intraperitoneal fatty implant around the cyst, thickening of peritoneum, diffuse wall thickening of small bowels, and increased amount of ascites. Acute or chronic peritonitis caused by rupture of an ovarian cystic teratoma can manifest as ascites, diffuse or focal omental infiltration, and inflammatory masses involving the omentum and bowel, which mimic peritoneal carcinomatosis and tuberculous peritonitis [4,9].
The treatment of choice, once rupture of an ovarian cystic teratoma is diagnosed, is surgical intervention. The cases of spontaneously ruptured ovarian cystic teratoma have a favorable prognosis if obvious intraoperative signs of peritonitis are not seen, because prompt removal of a spontaneously ruptured ovarian cyst with thorough peritoneal lavage is sufficient to prevent prolonged chemical peritonitis. However, there is very limited information in published literature on the management of chronic granulomatous peritonitis following rupture of an ovarian cystic teratoma. Although there are reports of chemical peritonitis caused by intraoperative rupture of an ovarian cystic teratoma that required reoperation [11-13], most cases could be managed conservatively if all the cyst contents were completely removed macroscopically during laparoscopy [14]. The use of systemic corticosteroids may improve postoperative resolution in chronic granulomatous peritonitis [15]. Koshiba [14] reported a case of chemical peritonitis caused by spontaneously ruptured ovarian teratoma which had been left untreated for >2 weeks, in which they used prednisolone and immunosuppressive drug, azathioprine, for 1 year after surgery and successfully controlled granulomatous peritonitis.
In conclusion, although spontaneous rupture of an ovarian mature cystic teratoma is a rare condition, the importance of correct diagnosis cannot be overemphasized. We presented the computed tomography images of a mature cystic teratoma obtained before and after rupture which provided useful objective information in diagnosing such case. In patients with signs of peritonitis after surgery of ovarian cystic teratoma, chemical peritonitis should be included in the differential diagnosis and the use of corticosteroids should be considered.
Figures and Tables
Fig. 1
(A) Computed tomography (CT) scan shows a left ovarian mass (arrow) with fat-fluid level with small amount of ascites. (B) CT scan after rupture shows a left ovarian mass (arrow) surrounded by fat globules, decreased amount of fat component within the cyst, thickening of peritoneum, diffuse wall thickening of small bowels, and increased amount of ascites.
References
1. Iwata A, Matsubara K, Umemoto Y, Hashimoto K, Fukaya T. Spontaneous rupture of benign ovarian cystic teratoma in a premenarcheal girl. J Pediatr Adolesc Gynecol. 2009. 22:e121–e123.
2. Peterson WF, Prevost EC, Edmunds FT, Hundley JM Jr, Morris FK. Benign cystic teratomas of the ovary; a clinico-statistical study of 1,007 cases with a review of the literature. Am J Obstet Gynecol. 1955. 70:368–382.
3. Fibus TF. Intraperitoneal rupture of a benign cystic ovarian teratoma: findings at CT and MR imaging. AJR Am J Roentgenol. 2000. 174:261–262.
4. Phupong V, Sueblinvong T, Triratanachat S. Ovarian teratoma with diffused peritoneal reactions mimicking advanced ovarian malignancy. Arch Gynecol Obstet. 2004. 270:189–191.
5. Quer EA, Dockerty MB, Mayo CW. Ruptured dermoid cyst of the ovary simulating abdominal carcinomatosis; report of case. Proc Staff Meet Mayo Clin. 1951. 26:489–498.
6. Chang YT, Lin JY. Intraperitoneal rupture of mature cystic ovarian teratoma secondary to sit-ups. J Formos Med Assoc. 2009. 108:173–175.
7. Bhatla N, Khanna R, Bhargava VL. Intraperitoneal rupture of benign cystic teratoma. Int J Gynaecol Obstet. 1993. 40:163–164.
8. Suprasert P, Khunamornpong S, Siriaunkgul S, Phongnarisorn C, Siriaree S. Ruptured mature cystic teratomas mimicking advanced stage ovarian cancer: a report of 2 cases study. J Med Assoc Thai. 2004. 87:1522–1525.
9. Park SB, Kim JK, Kim KR, Cho KS. Imaging findings of complications and unusual manifestations of ovarian teratomas. Radiographics. 2008. 28:969–983.
10. Nitinavakarn B, Prasertjaroensook V, Kularkaew C. Spontaneous rupture of an ovarian dermoid cyst associated with intra-abdominal chemical peritonitis: characteristic CT findings and literature review. J Med Assoc Thai. 2006. 89:513–517.
11. Achtari C, Genolet PM, Bouzourene H, De Grandi P. Chemical peritonitis after iatrogenic rupture of a dermoid cyst of the ovary treated by coelioscopy. Apropos of a case and review of the literature. Gynakol Geburtshilfliche Rundsch. 1998. 38:146–150.
12. Clement D, Barranger E, Benchimol Y, Uzan S. Chemical peritonitis: a rare complication of an iatrogenic ovarian dermoid cyst rupture. Surg Endosc. 2003. 17:658.
13. Rubod C, Triboulet JP, Vinatier D. Ovarian dermoid cyst complicated by chemical peritonitis: case report. Gynecol Obstet Fertil. 2007. 35:651–653.
14. Koshiba H. Severe chemical peritonitis caused by spontaneous rupture of an ovarian mature cystic teratoma: a case report. J Reprod Med. 2007. 52:965–967.
15. Stuart GC, Smith JP. Ruptured benign cystic teratomas mimicking gynecologic malignancy. Gynecol Oncol. 1983. 16:139–143.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download