Abstract
Acute aortic dissection may indeed be a rare complication of pregnancy, and the majority of aortic dissections usually occur during the third trimester of pregnancy. Most aortic dissections occur as a result of systemic hypertension or connective tissue disorders. Without any treatment, more than 50% of patients die within the initial 48 hours, and the 3-month mortality rate is as high as 90%. Because the pain in puerperal women is uncertain and difficult to discriminate from physiologic pain, the diagnosis of aortic dissection is complex and requires a high index of suspicion. We experienced a case of unexpected acute aortic dissection that occurred after elective cesarean section delivery in a young woman without any known risk factors. This case of aortic dissection was managed medically. In postpartum patients complaining of chest pain, the suspicion of aortic dissection is important for prompt diagnosis and better prognosis.
Acute aortic dissection is a life-threatening disease that is rare in young women. Aortic dissection occurs approximately 2-3 times as often in men as in women between the ages of 50 and 70 years old. Most aortic dissections occur as a result of systemic hypertension. Additional risk factors, such as Marfan's syndrome, Ehlers-Danlos syndrome, aortitis, coarctation of the aorta, bicuspid aortic valve, cocaine abuse, trauma, and pregnancy have also been identified [1]. Aortic dissection occurs especially in women below the age of 40 years old in pregnancy related cases [2].
Prompt diagnosis is crucial because of the increased risk of maternal mortality. However, owing to the low incidence, the diagnosis of acute aortic dissection in young women might be missed or delayed in patients who have neither risk factors nor typical clinical manifestations. Here, we present a case of unexpected acute aortic dissection that occurred after elective cesarean section delivery in a young woman without any known risk factors. This case of aortic dissection was managed medically.
A 30-year-old woman was referred to the emergency room of our institute with severe acute chest pain occurred during breast feeding. This pain had been occurring for 2 hours. The patient had no history of chronic hypertension, cardiac disease, kidney disease, or connective tissue disorder. There was no family history of Marfan's syndrome and no history of trauma, smoking, drug or acohol abuse. She had given birth to a healthy 3,250 g boy by elective cesarean section 4 days before.
The cesarean section was performed due to a history of myomectomy 4 years before. This birth was her first pregnancy, and she had undergone routine antenatal care without any problems. Her blood pressure ranged between 130/80-110/60 mm Hg in the operation period and was in the range of the 110/70 mm Hg in the postpartum period. However, after severe acute chest pain occurred, her blood pressure ranged from 160/90 to 190/110 mm Hg.
On admission, the patient described a sharp and persistent chest pain accompanied by shortness of breath, but without nausea, vomiting, or diaphoresis. The patient's blood pressure was 190/110 mm Hg, heart rate was 92 beats per minute and regular, respiratory rate was regular at 24 breaths per minute, and body temperature was 36.7℃. The patient weighed 70.5 kg with a height of 162.5 cm. The examinations of her skeleton, eye, lung, and skin were normal and the rest of the physical examination was normal. Laboratory examinations revealed a white blood cells count of 5,310/mm3, a hematocrit level of 31.1%, an erythrocyte sedimentation rate at 22 mm/hr, a total cholesterol level of 262 mg/dL, a triglyceride level of 217 mg/dL, a low-density lipoprotein level of 113 mg/dL. The coagulation test results, urinalysis, arterial blood gases analyses, D-dimer, and cardiac enzyme levels were normal. The rapid plasma reagin antibody was negative.
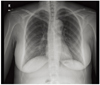
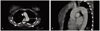
An electrocardiogram and chest X-ray were performed, and the results were normal (Fig. 1). The patient's normal electrocardiogram and cardiac enzyme levels excluded a myocardial infarction. Normal coagulation test results and D-dimer level made pulmonary embolism unlikely. However, there was no improvement in the chest pain or the hypertension. Under the suspicion of aortic dissection, a computed tomographic angiography scan was performed. The interpretation of the scan confirmed the diagnosis of DeBakey type III aortic dissection that extended from the proximal descending aorta to the left common iliac artery (Fig. 2). The maximal diameter of the descending aorta was 3.9 cm and there was no involvement of renal and ovarian artery.
The patient was admitted to the cardiology intensive care unit (CCU) with cardiology consultation. A bedside trans-esophageal echocardiography demonstrated aortic dissection beginning just distal to the opening of the left subclavian artery and extending down to the abdominal aorta. The dissection showed communication from the true lumen to the false lumen through an approximately 0.6 cm sized tear site, which was about 1.2 cm distal to the opening of the left subclavian artery. The patient's left ventricular ejection function was intact, with no evidence of aortic regurgitation. The patient received intravenous antihypertensive medications consisting of β-blockers (labetalol 500 mg, esmolol 500 mg a day) and nitroprusside (nirtroprusside 50 mg a day). On the third day after admission to the CCU, the patient's blood pressure was normalized and she no longer complained of chest pain. The patient was then transferred to the general ward, and her blood pressure was controlled by oral medications consisting of a β-blocker (atenolol 50 mg a day), a calcium channel blocker (nifedipine 40 mg a day), and an angiotensin converting enzyme inhibitor (perindopril tetrabutylamine 4 mg a day).
The patient was discharged on the ninth day after admission in good physical health and with normal blood pressure by means of oral medications. One-month and 3-month follow-up computed tomographic angiographies revealed that the aortic dissection was not further aggravated and a 6-month follow-up indicated that her blood pressure was stable and surgical intervention was not necessary.
Acute aortic dissection is a rare, but potentially catastrophic complication of pregnancy. Aortic dissection usually occurs in the third trimester when blood volumes and cardiac output are maximally rising. It has also been known to occur at all stages of pregnancy and during the weeks after delivery [3].
Chronic hypertension is present in 70-90% of patients with aortic dissection [1]. During pregnancy, there is an increase in heart rate, stroke volume, cardiac output, left ventricular wall mass, and end-diastolic dimensions [4]. These hemodynamic states are similar to those in chronic hypertension. The gravid uterus has been reported to cause significant compression of the aorta and iliac arteries, especially in the supine position, and this may increase the outflow resistance of the lower arterial trees [5]. The concurrent increase in cardiac ejection into the upper aorta may further predispose the patient to the initiation of an intimal tear, causing aortic dissection [6]. Because these modifications occur in every pregnancy and return to the pre-pregnancy state rapidly after parturition, it is unlikely that cardiovascular and hemodynamic changes alone cause the damage to the aorta, even in puerperium. Hormonal factors may also be related to the occurrence of aortic dissection. A programmed dilatation of the aorta, the renal and placental resistance vessels, and the venous system has been observed in pregnancy. This improves the perfusion of the pregnant body. Specific receptors for estrogen and progesterone increase throughout pregnancy, and then fall back to pre-pregnancy levels after parturition [7]. These hormonal effects lead to a fragmentation of the reticulin fibers, diminish the amount of acid mucopolysaccharides, and favor the loss of normal corrugation of the elastic fibers [8]. However, as these changes in hormonal factors occur during every pregnancy, it is hard to identify close correlations between aortic dissection and hormonal effects during pregnancy. Other risk factors described in pregnancy include Marfan's syndrome, Ehlers-Danlos syndrome, aortitis, coarctation of the aorta, bicuspid aortic valve, cocaine abuse, and trauma [1]. The patient reported here was known to have had normal blood pressure throughout her pregnancy and did not have any risk factors. The patient did not meet the revised diagnostic criteria for the Marfan's syndrome proposed by De Paepe et al. [9] of at least three body systems affected, with at least two systems showing clear physical signs relatively specific for the syndrome in patients with no family history of the disorder. In this criteria, the body systems include the skeletal, ocular, cardiovascular, pulmonary, skin and integument, and dural [9]. It is unfortunate that we could not clearly identify the etiology of the aortic dissection in our patient.
Our patient presented with severe acute chest pain, which was not relieved by potent narcotics. About 96% of individuals with aortic dissection present with severe pain that had a sudden onset. It may be described as tearing, stabbing or sharp in nature. 17% of individuals will feel the pain migrate as the dissection extends down the aorta. The location of pain is associated with the location of the dissection. Individuals suffering from an aortic dissection usually do not present with diaphoresis [1]. On physical examination, blood pressure may increase or decrease. Neurologic deficits are a presenting sign in up to 20% of cases [10].
The differential diagnoses of severe chest pain that is not relieved by narcotics include acute myocardial infarction, pulmonary embolism, and aortic dissection. The presence of a normal electrocardiogram and normal cardiac enzymes were helpful in excluding myocardial infarction. Normal coagulation test results and D-dimer level helped rule out pulmonary embolism. Moreover, because the pain in puerperal women is uncertain and difficult to discriminate from physiologic pain, the differential diagnosis is complex and requires a high index of suspicion.
Once a diagnosis of aortic dissection is entertained, common tests used to confirm the diagnosis include a computed tomographic angiography scan and a trans-esophageal echocardiogram. The trans-esophageal echocardiogram (TEE) is a relatively good test for the diagnosis of aortic dissection, with a sensitivity of up to 98% and a specificity of up to 97%. Disadvantages of TEE include the inability to visualize the distal ascending aorta (the beginning of the aortic arch) and the descending abdominal aorta, which lies below the stomach [11]. Computed tomographic angiography is a fast, non-invasive test that provides an accurate three-dimensional view of the aorta. This test has a sensitivity of 96-100% and a specificity of 96-100%. One disadvantage is the need for iodinated contrast. Other tests that may be used include an aortogram or magnetic resonance angiogram of the aorta. The use of contrast dyes makes the computed tomographic angiography scan and aortogram less favorable during pregnancy [11,12].
Aortic dissection is classified using the DeBakey system and the Stanford system. According to the DeBakey classification system, aortic dissection is categorized into three types based on where the original intimal tear is located and the extent of the dissection. Type I aortic dissection originates in the ascending aorta, propagates at least to the aortic arch, and often extends distally. Type II aortic dissection is confined to the ascending aorta. Type III aortic dissection originates in the descending aorta and rarely extends proximally. The Stanford classification system divides aortic dissection into two groups depending on whether the ascending aorta is involved. Type A is comparable to types I and II in the DeBakey system, and type B is equivalent to type III in the DeBakey system. When the aortic dissection incidence rates during pregnancy were examined based on classification type, the incidence of Stanford type A aortic dissection was 89% (DeBakey type I 70%, DeBakey type II 19%) and that of Stanford type B (DeBakey type III) aortic dissection was 11% [13].
Acute aortic dissection is a life-threatening event. Without treatment, more than 50% of patients die within the initial 48 hours, and the 3-month mortality rate is as high as 90% [12]. The most common cause of death is aortic rupture into the pericardium resulting in cardiac tamponade and neurologic abnormalities have also been described in some survivors [10].
In the case of an acute aortic dissection, once diagnosis has been confirmed, the choice of treatment depends on the location of the dissection. For an ascending aortic dissection, surgical management is superior to medical management. On the other hand, in the case of an uncomplicated distal aortic dissection (including abdominal aortic dissections), medical management is preferred over surgical treatment. The prime consideration in the medical management of aortic dissection is strict blood pressure control. Another factor is the need to reduce the shear-force (force of blood ejection from the left ventricle). To reduce shear stress, a vasodilator such as sodium nitroprusside should be used with β-blockers such as esmolol, propranolol, or labetalol. The β-blocking properties of labetalol make it especially attractive in this situation. Calcium channel blockers can also be used in the treatment of aortic dissection, particularly if there is a contraindication to the use of β-blockers or if the individual has refractory hypertension [14]. During pregnancy, β-blockers are the drug of choice. Sodium nitroprusside is less desirable because of the risk of fetal cyanide poisoning [15]. When medical therapy fails or when the clinical presentation is severe and deteriorating, immediate surgical repair should be considered [6].
This case suggests that acute aortic dissection can present with non-specific clinical symptoms in young, normotensive and postpartum women unexpectedly. We have to consider aortic dissection when a puerperal woman complains of chest pain even if she has no history of personal or familial chronic hypertension or connective tissue disorders.
Figures and Tables
References
1. Burchell HB. Aortic dissection (dissecting hematoma; dissecting aneurysm of the aorta). Circulation. 1955. 12:1068–1079.
2. Oskoui R, Lindsay J Jr. Aortic dissection in women < 40 years of age and the unimportance of pregnancy. Am J Cardiol. 1994. 73:821–823.
3. Immer FF, Bansi AG, Immer-Bansi AS, McDougall J, Zehr KJ, Schaff HV, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003. 76:309–314.
4. Barrett JM, Van Hooydonk JE, Boehm FH. Pregnancy-related rupture of arterial aneurysms. Obstet Gynecol Surv. 1982. 37:557–566.
5. Ohlson L. Effects of the pregnant uterus on the abdominal aorta and its branches. Acta Radiol Diagn (Stockh). 1978. 19:369–376.
6. Wheat MW. Dorghazi S, editor. Intensive drug therapy. Aortic dissection. 1983. New York (NY): McGraw-Hill;55–60.
7. Leiberman JR, van Vroonhoven CC, Beckmann I, van der Kwast TH, Wallenburg HC. Uterine artery estrogen receptors in the nonpregnant and pregnant guinea pig. Am J Obstet Gynecol. 1990. 163:1685–1688.
8. Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol. 1967. 83:336–341.
9. De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996. 62:417–426.
10. DeSanctis RW, Doroghazi RM, Austen WG, Buckley MJ. Aortic dissection. N Engl J Med. 1987. 317:1060–1067.
11. Ergin MA, Lansman SL, Griepp RB. Baue AE, Geha AS, Hammond GL, Laks H, Naunheim KS, editors. Dissections of aorta. Glenn's thoracic and cardiovascular surgery. 1991. 5th ed. Norwalk (CT): Appleton & Lange;1955–1961.
12. Scott C, Burruss N, Kalimi R, Manetta F, Palazzo RS, Graver LM. Acute ascending aortic dissection during pregnancy. Am J Crit Care. 2001. 10:430–433.
13. Konishi Y, Tatsuta N, Kumada K, Minami K, Matsuda K, Yamasato A, et al. Dissecting aneurysm during pregnancy and the puerperium. Jpn Circ J. 1980. 44:726–733.
14. Walker PJ, Sarris GE, Miller DC. Rutherford RB, editor. Peripheral vascular manifestations of acute aortic dissection. Vascular surgery. 1995. 4th ed. Philadelphia (PA): W.B. Saunders;1087–1102.
15. Rutherford RB, Nolte JE. Aortic and other arterial dissections associated with pregnancy. Semin Vasc Surg. 1995. 8:299–305.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download