Abstract
Objective
The aim of this study is to assess the risk of adverse pregnancy outcome by maternal serum markers in Quad test and to compare the existing cutoff (2.0 MoM) with 95 percentile cutoff generated from our consecutive population in predicting adverse pregnancy outcome.
Methods
We generated 95 percentile cutoff as our own reference using 3,000 consecutive women who performed Quad test. Except for follow-up loss, fetal aneuploidy and anomaly, 2,598 women were analyzed for the assessment of adverse pregnancy outcome consisted of preeclampsia (PE), preterm birth (<32 weeks), low birth weight and fetal death in utero.
Results
We confirmed high levels of alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), and inhibin A are associated with development of PE, preterm birth, and low birth weight. In general, 95 percentile cutoff corresponded similarly with 2.0 MoM except AFP. Combination of serum markers increased the odds ratio for predicting adverse pregnancy outcome. The women with higher level of both AFP and inhibin A (2.0 ≥ MoM) had 9.8 times higher risk for PE, 24.8 times higher risk for preterm birth, and 5.6 times higher risk for low birth weight. The women with higher level of AFP, hCG, and inhibin A had 8.2 times higher risk for PE, 29.4 times higher risk for preterm birth. Notably, negative predictive value of serum markers for PE and preterm birth are over 98% either in alone and in combination.
REFERENCES
1. Dugoff L. Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010; 115:1052–61.

2. Krause TG, Christens P, Wohlfahrt J, Lei U, Westergaard T, N⊘rgaard-Pedersen B, et al. Second-trimester maternal serum alpha-fetoprotein and risk of adverse pregnancy outcome (1). Obstet Gynecol. 2001; 97:277–82.
3. Yaron Y, Cherry M, Kramer RL, O'Brien JE, Hallak M, Johnson MP, et al. Second-trimester maternal serum marker screening: maternal serum alpha-fetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol. 1999; 181:968–74.
4. Muttukrishna S. Role of inhibin in normal and high-risk pregnancy. Semin Reprod Med. 2004; 22:227–34.

5. Muttukrishna S, Knight PG, Groome NP, Redman CW, Ledger WL. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet. 1997; 349:1285–8.

6. Dugoff L, Hobbins JC, Malone FD, Vidaver J, Sullivan L, Canick JA, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005; 106:260–7.

7. Berkeley AS, Killackey MA, Cederqvist LL. Elevated maternal serum alpha-fetoprotein levels associated with breakdown in fetal-maternal-placental barrier. Am J Obstet Gynecol. 1983; 146:859–61.
8. Perkes EA, Baim RS, Goodman KJ, Macri JN. Second-trimester placental changes associated with elevated maternal serum alpha-fetoprotein. Am J Obstet Gynecol. 1982; 144:935–8.
9. Chandra S, Scott H, Dodds L, Watts C, Blight C, Van Den Hof M. Unexplained elevated maternal serum alpha-fetoprotein and/or human chorionic gonadotropin and the risk of adverse outcomes. Am J Obstet Gynecol. 2003; 189:775–81.
10. Spencer K. Second-trimester prenatal screening for Down syndrome and the relationship of maternal serum biochemical markers to pregnancy complications with adverse outcome. Prenat Diagn. 2000; 20:652–6.

11. Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Canick JA, Haddow JE, et al. Maternal serum unconjugated oestriol as an antenatal screening test for Down's syndrome. Br J Obstet Gynaecol. 1988; 95:334–41.

12. Schoen E, Norem C, O'Keefe J, Krieger R, Walton D, To TT. Maternal serum unconjugated estriol as a predictor for Smith-Lemli-Opitz syndrome and other fetal conditions. Obstet Gynecol. 2003; 102:167–72.

13. Oh HM, Kim SY, Lee SP, Sohn YK, Lee GH, Kim JM. The association between adverse pregnancy outcomes and the serum inhibin A levels in mid-trimester of gestation. Korean J Obstet Gynecol. 2008; 51:1239–44.
14. Jelliffe-Pawlowski L, Baer R, Moon-Grady AJ, Currier RJ. Second trimester serum predictors of congenital heart defects in pregnancies without chromosomal or neural tube defects. Prenat Diagn. 2011; 31:466–72.

15. Zhong Y, Bradshaw R, Stanley AP, Odibo AO. The impact of assisted reproductive technology on the association between first-trimester pregnancy-associated plasma protein a and human chorionic gonadotropin and adverse pregnancy outcomes. Am J Perinatol. 2011; 28:347–54.

16. Health Insurance Review & Assessment Service [Internet]. Seoul (KR): Health Insurance Review & Assessment Service;c2010. [cited 2011 Oct 15]. Available from:. http://www.hira.or.kr/.
17. Aquilina J, Thompson O, Thilaganathan B, Harrington K. Improved early prediction of pre-eclampsia by combining second-trimester maternal serum inhibin-A and uterine artery Doppler. Ultrasound Obstet Gynecol. 2001; 17:477–84.

Fig. 1.
ROC curve comparing maternal serum marker for prediction of adverse pregnancy outcome. FDIU, fetal death in utero; ROC, receiver operator characteristic; AUROC, area under receiver operator characteristic; AFP, alpha-fetoprotein; uE3, unconjugated estriol; hCG, human chorionic gonadotropin.
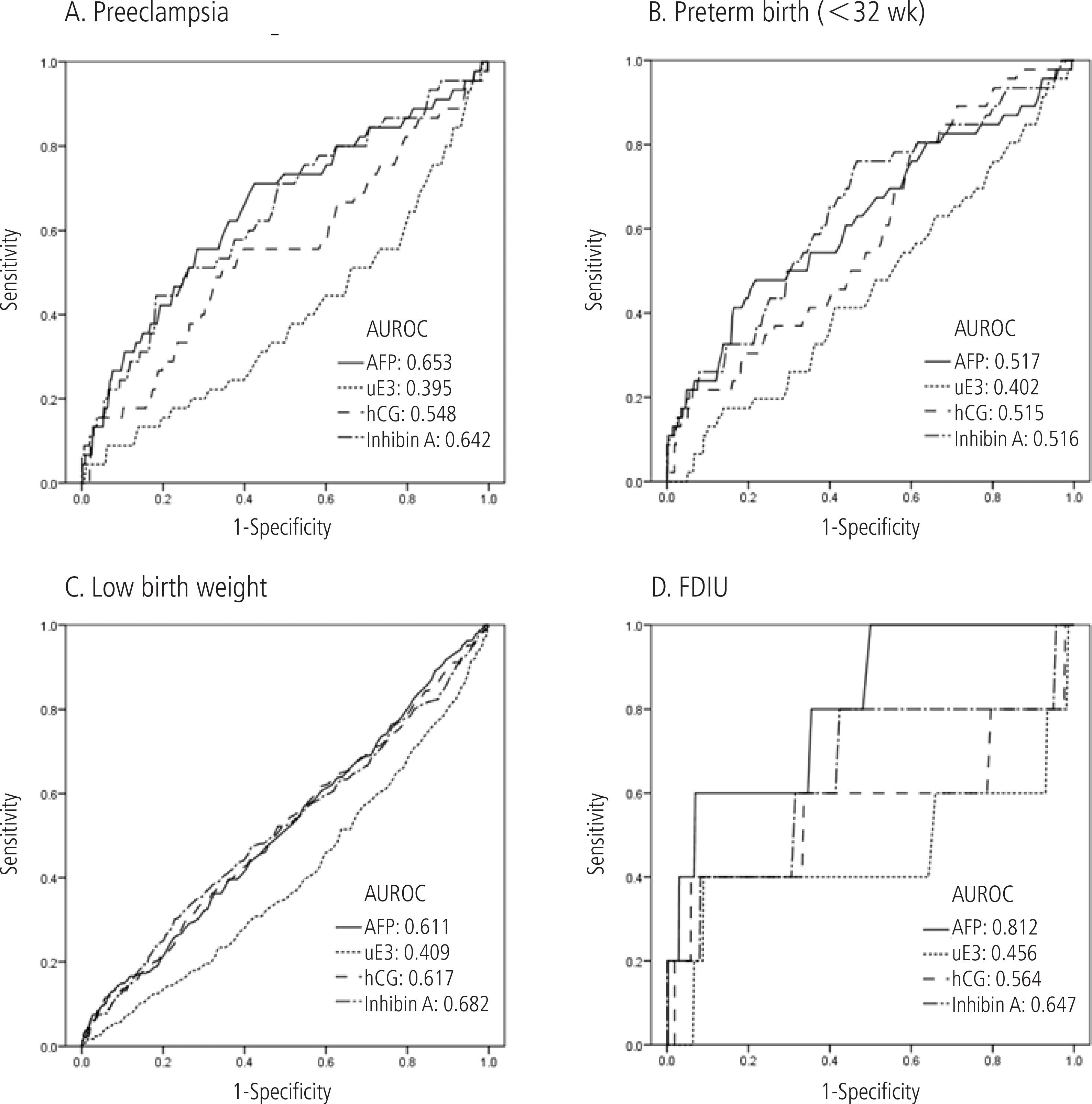
Table 1.
Distribution of abnormal serum marker combination (n=2,598)
Table 2.
Correlation between all serum markers
Table 3.
Association between selected serum markers and adverse pregnancy outcomes
Table 4.
Diagnostic value of individual and combination of serum markers interpretation of adverse pregnancy outcomes
PPV, positive predictive value; NPV, negative predictive value; OR, odds ratio; CI, confidence interval; AFP, AFP ≥ 2.0 MoM; hCG, hCG ≥ 2.0 MoM; Inh, Inhibin A ≥ 2.0 MoM; AFP + Inh, AFP ≥ 2.0 MoM and Inhibin A ≥ 2.0 MoM; hCG + Inh, hCG ≥ 2.0 MoM and Inhibin A ≥ 2.0 MoM; AFP + hCG + Inh, AFP ≥ 2.0 MoM and hCG ≥ 2.0 MoM and Inhibin A ≥ 2.0 MoM.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download