Abstract
The carcinoma of the uterine cervix in virgins is extremely rare. A 36-year-old virgin presented with a 4.8 × 2.2 cm sized endocervical mass with bleeding on sonography. Biopsy revealed a squamous cell carcinoma arising in the uterine cervix without human papilloma virus (HPV) DNA. Positron emission tomography revealed cervical cancer with multiple hematogenous lung metastases. After 3 cycles of preoperative chemotherapy, she underwent modified radical hysterectomy and 6 cycles of postoperative chemotherapy. After the treatment, complete resolution was obtained, and she has been followed without recurrence for 4 years. Although > 90% of cervical cancer is caused by HPV infection, we cannot completely deny the possibility of HPV negative cervical carcinoma.
Cervical cancer is one of the most prevalent cancer in women in South Korea, and infection with oncogenic human papilloma virus (HPV) is believed as the direct causative factor [1]. The International Biological Study on Cervical Cancer (IBSCC) conducted a study of invasive cervical cancers and reported a worldwide HPV prevalence of 93 percent [2]. Then, Walboomers et al. [3] reanalyzed HPV-negative cases from the IBSCC study and claimed that the worldwide HPV prevalence in cervical carcinomas was 99.7 percent. However, even with this prevalence, the possibility of HPV unrelated carcinomas still needs to be considered.
We performed a literature search in Ovid MEDLINE and PubMed with key words, "cervical cancer, and/or virgin, and/or HPV negative" and identified only 4 cases reported between January 1966 and July 2008 [4,5]. Here we report one case of HPV negative, squamous cell carcinoma of the uterine cervix with diffuse hematogenous lung metastasis in a 36-year-old woman whose hymen was completely intact.
In November 2007, a 36-year-old, never married nulligravida was referred to the National Health Insurance Corporation Ilsan Hospital for further evaluation and treatment of intractable vaginal bleeding which lasted for about 20 days. Because she claimed that she never had sexual experience and her hymen was completely intact on physical examination, we performed transrectal ultrasonography to investigate the source of the vaginal bleeding. The ultrasonography and magnetic resonance imaging with contrast enhancement showed a 4.8 × 2.2 cm sized mass, dilating the endocervical canal, and it was suspected to be endometrial carcinoma (Fig. 1).
The medical, surgical, and menstrual history of the patient was unremarkable. She had been well and healthy until the bleeding started in October 2007. After the bleeding started, she first visited a private clinic where an endometrial mass measured by 5 × 2 cm was noted on transrectal ultrasonography. She was prescribed oral contraceptives, but the symptom persisted.
Although she was a virgin, we decided to carry out endometrial curettage because a malignant lesion was so strongly suspected that histological confirmation was warranted. Moreover, her bleeding had not stopped. Endometrial curettage was performed under the patient's consent. During the curettage, we noticed that her uterine cervix on the speculum was orange colored, easily bleed, and fragile, which are all suggestive of classical squamous cell carcinoma of the cervix. A frozen section of the fractional curettage confirmed that she had squamous cell carcinoma of the uterine cervix, not endometrial adenocarcinoma.
She was admitted for the baseline workup, including positron emission tomography (PET), colonoscopy, intravenous pyelogram (IVP), and cystoscopy. PET scan revealed 5 cm sized carcinoma with multiple hematogenous lung metastases (Fig. 2). However, there were no lesions suggestive of lymph node metastasis or local pelvic extension. The cancer mass appeared to be absolutely confined to the uterine cavity except the hematogenous lung metastasis. Cystoscopy, IVP, and colonoscopy results were not remarkable.
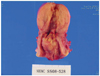
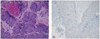
After three cycles of preoperative adjuvant chemotherapy with cisplatin (50 mg/m2) and vincristine (1 mg/m2) per week, she underwent modified radical hysterectomy, ovarian transposition, bilateral pelvic lymph node dissection, and para-aortic lymph node sampling. Grossly, the cervix revealed a large tumorous mass, measuring 4.5 × 3.2 cm in size at the posterior wall. The cut section of the mass was yellow-gray, firm, and necrotic. The mass was extended up to the serosa of the posterior wall and focally into endometrial cavity (Fig. 3). The uterine cavity measured 3 cm long, and the endometrium mass irregularly thickened. Microscopically, the tumor mass showed histologic features of typical squamous cell carcinoma with individual keratinization (Fig. 4A). Tumor cells reached the endometrium but were confined to the uterus. No lymph node metastasis or parametrial involvement was found. Tumor cells were strongly positive for p53 in immunohistochemical assay (Fig. 4B).
The patient had no postoperative complications and underwent six cycles of postoperative adjuvant chemotherapy with carboplatin and paclitaxel. Surprisingly, her multiple hematogenous lung metastatic nodules were completely resolute after three cycles of chemotherapy, and she recovered complete resolution after completing all six cycles of chemotherapy. She has been followed in the out-patient department without recurrence for 4 years.
This HPV genome negative squamous cell carcinoma developed in a virgin is a very interesting case in terms of its extreme rarity and atypical clinical course.
This case is directly contrary to our belief that virtually all cervical carcinoma is caused by sexually transmitted HPV. A few possible carcinogenetic mechanisms could be suggested to explain this rare case.
First, this may be a case with a failure to identify HPV. Integration of HPV DNA in the host cell genome could result in disruption, combined with deletions, of HPV DNA at integration sites [6]. However, modern PCR technologies are too advanced to detect HPV DNA at the single copy level even in an overwhelming background of human DNA. Therefore, this explanation is not likely to be valid in our case.
Second, there are pathways which might give rise to cervical carcinoma in which HPV is transiently involved, thus called a "hitand-run" mechanism. However, the "hit-and-run" mechanism is unlikely, because transcription of E6 and E7 oncoproteins of HRHPV is required for the maintenance of epithelial transformation of the uterine cervix [3,7,8]. Actually, only one case appeared to be related with this mechanism [9].
Finally, carcinoma of the cervix might develop through truly HPVindependent pathways. This speculation is supported by an epidemiological finding that patients with HPV-negative cervical intraepithelial neoplasia (CIN) have a different spectrum of risk factors from those with HPV-positive CIN [9]. One of the target molecule related with this HPV-independent carcinogenesis of uterine cervix is p53. Although most p53 dysfunctions in cervical cancer is related with E6 oncoprotein of high risk (HR)-HPV, other mechanisms such as point mutation or genetic polymorphism [10,11] could occur through a HPV-independent process, and initiate the malignant transformation. In our case, p53 mutation was shown by immunohistochemical study (Fig. 4B). Based on this finding, we speculated that this HPV-negative cervical cancer arose through de novo mutation of p53, without HR-HPV DNA.
Moreover, this case had diffuse hematogenous lung metastasis. Generally, the cervical cancer progresses in a "level by level" fashion to regional nodes through the lymphatic channels, and also to extra-nodal sites via the hematogenous stream [12]. However, if it was for pulmonary metastasis, the clinical stage of this case was just Ib2. The lesion was strictly confined to the uterine cervix, and there was no demonstrable pelvic and paraaortic lymph node metastasis. Our case showed a very early and unique (hematogenous, rather than lymphatic) pattern of metastasis compared with those of typical carcinoma of the cervix. It might be attributed to de novo p53 mutation that was already described. In many other malignancies, the p53 mutation is related with poor prognosis such as locally advanced lesion, multiple lymph node metastasis, and distant solid organ metastasis. However, the value of p53 mutation as a prognostic marker in cervical cancer is not so useful [13] because the E6 of the HR-HPV basically initiates carcinogenesis by ubiquitin-dependent degradation of p53 [8,14,15]. However, if this p53 overexpression is initiated by a de novo process, the prognosis could be different from that of typical HPV-related cervical cancer.
Generally, it is difficult to recommend transvaginal procedures such as PAP smear, colposcopy or curettage to patients who claim to have never had sexual intercourse. Many clinicians regard cervical cancer as virtually nonexclusively caused by HR-HPV infection. In these aspects, cervical cancer in virgins might be neglected and diagnosed in late stage. Therefore, clinicians should make a clear decision over when to use invasive procedures, and always have suspicion about the possibilities of rare occasions.
Figures and Tables
Fig. 1
Magnetic resonance imaging demonstrated a 4.8 × 2.2 cm sized mass and it was suspected to be endometrial carcinoma.

References
1. Bosch FX, de Sanjosé S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers. 2007. 23:213–227.
2. Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995. 87:796–802.
3. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999. 189:12–19.
4. Wang IT, Chang CW, Tang WL, Chen WY, Liu WM. Keratinizing squamous cell carcinoma of the cervix in a sexually inexperienced woman: a case report. J Reprod Med. 2006. 51:745–746.
5. Bielecki A, Borowicz K, Misiewicz L. Adenocarcinoma of the cervix uteri in a 37-year-old virgin with coexisting cancer of the body of the uterus. Am J Obstet Gynecol. 1963. 87:135–136.
6. Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985. 314:111–114.
7. Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007. 98:1505–1511.
8. Wise-Draper TM, Wells SI. Papillomavirus E6 and E7 proteins and their cellular targets. Front Biosci. 2008. 13:1003–1017.
9. Burger MP, Hollema H, Pieters WJ, Schröder FP, Quint WG. Epidemiological evidence of cervical intraepithelial neoplasia without the presence of human papillomavirus. Br J Cancer. 1996. 73:831–836.
10. Park DJ, Wilczynski SP, Paquette RL, Miller CW, Koeffler HP. p53 mutations in HPV-negative cervical carcinoma. Oncogene. 1994. 9:205–210.
11. Min-min H, Ming-rong X, Ze-yi C, Kai-xuan Y, Zhi-lin S. Analysis of p53 codon 72 polymorphism and its association with human papillomavirus 16 and 18 E6 in Chinese cervical lesions. Int J Gynecol Cancer. 2006. 16:2004–2008.
12. Belhocine T, Kridelka F, Thille A, De Barsy C, Foidart-Willems J, Hustinx R, et al. Staging of primary cervical cancers: the role of nuclear medicine. Crit Rev Oncol Hematol. 2003. 46:275–284.
13. Lindstrom AK, Stendahl U, Tot T, Lidström BM, Hellberg D. Predicting the outcome of squamous cell carcinoma of the uterine cervix using combinations of individual tumor marker expressions. Anticancer Res. 2007. 27:1609–1615.
14. Braun K, Ehemann V, Waldeck W, Pipkorn R, Corban-Wilhelm H, Jenne J, et al. HPV18 E6 and E7 genes affect cell cycle, pRB and p53 of cervical tumor cells and represent prominent candidates for intervention by use peptide nucleic acids (PNAs). Cancer Lett. 2004. 209:37–49.
15. Nair S, Pillai MR. Human papillomavirus and disease mechanisms: relevance to oral and cervical cancers. Oral Dis. 2005. 11:350–359.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download