Abstract
A case of a moderate differentiated endometrial carcinoma of the uterus with a synchronous poorly differentiated bladder cancer is reported. A review of the literature revealed that simultaneous presentation of primary endometrial and bladder neoplasm is rare and usually related to low-stage bladder lesions in contrast to our case with undifferentiated and the deep myometrial invasion of bladder lesion. A 79-year-old woman with endometrial cancer stage IB was performed of total abdominal hysterectomy with bilateral salpingo-oophorectomy, pelvic and para-aortic lymph nodes dissection and adjuvant concurrent cisplatin-radiation therapy. After treatment, she complained intermittent gross hematuria. She was performed the bladder mucosal biopsy and finally diagnoses with poorly differentiated carcinoma of bladder. She received transurethral resection of bladder tumor alone without total cystectomy or any other adjuvant treatment due to her refusal. Her condition is tolerable except intermittent hematuria and anemia.
In the case of synchronous detection of neoplasms of two organs, the distinction between double primary tumors with metastasis to the other organs based on conventional clinicopathologic criteria may be difficult. The distinction between metastatic and double primary tumors is significant since it affects staging and prognosis [1]. We reported the rare case of endometrium-bladder double primary cancer, which showed the presence of features of poorly differentiated carcinoma of the bladder along with deep invasion of the myometrium, contrary to early stage of endometrial cancer.
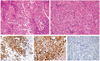
A 79-year-old (gravid 3, para 3) woman was admitted in our department with a 3-month history of irregular vaginal spotting and bleeding accompanied by frequency. In the past history, other than total hip replacement surgery received 15 years ago, special findings were not observed. In pelvic examination, compared to her ages the uterus was large and soft. In sonogram the endometrial thickness was 2.4 cm. She was performed endometrial biopsy and diagnosed moderately differentiated endometrioid carcinoma. Magnetic resonance imaging confirmed the existence of nonhomogeneous endometrial tumor which invaded the myometrium more than half (Fig. 1A). There was no evidence of retroperitoneal lymph node or liver involvement. Her clinical stage was endometrial cancer Ib. Complete blood count, renal and liver function tests were unremarkable. Serum tumor markers were elevated (CA-125, 65.3 IU/mL). At laparotomy, the uterus was enlarged with endometrial tumor measuring 2.5×2.7 cm and a diffusely enlarged uterus were discovered. Both ovaries appeared normal as did the omentum and the other pelvic and abdominal viscera. Total hysterectomy and bilateral salpingo-oophorectomy as well as pelvic and paraaortic lymphadenectomy were performed. Histopathologic examination disclosed moderately differentiated endometrioid carcinoma of the endometrium with myometrial invasion more than half (Fig. 2A). Histopathologic examination of the omentum was unremarkable. Cytology of peritoneal washings did not reveal malignant cells. She received the adjuvant concurrent cisplatin-radiation therapy. After finished the treatment she followed up endometrial cancer with positron emission tomography-computed tomography (PET-CT) and tumor marker in 3 months later. Tumor marker, CA-125 was within normal range and there was no evidence of malignancy in PET-CT scan. However she continuously complained frequency and intermittent gross hematuria. We performed the bladder mucosal biopsy under cystoscopy and finally diagnoses with poorly differentiated urothelial carcinoma with sarcomatous variant of bladder (Fig. 2B). We also performed the immunohistochemistry stain to make sure whether the bladder carcinoma was metastatic tumor from endometrium or not. Among the cytokeratin (CK)-7, CK20 and vimentin staining the tumor showed strong positive in vimentin and focal positive in CK7 (Fig. 2C-2E). The patient did not want to receive further evaluation nor treatment for bladder cancer. She was only performed the transurethral resction-bladder tumor (TUR-BT) to control the gross hematuria and obstructive symptoms. Three months later bladder tumor was grown again. She was performed TUR-BT twice more and now follow-up with conservative management about anemia (Fig. 3).
The simultaneous appearance of primary ovarian and endometrial carcinoma is relatively rare (0.3% of the genital tract malignancies) [2]. In the case of synchronous detection of neoplasms of endometrium and ovary with similar histologic features, the distinction between double primary tumors or ovarian cancer with metastasis to the endometrium or vice versa based on conventional clinicopathologic criteria may be difficult. In the case of tumors with dissimilar histology, the diagnosis of two synchronous primary tumors is less in doubt. As shown by Castro et al. [3] following a literature review, tumors with dissimilar histology comprise 27.2% of cases of simultaneous carcinomas of endometrium and ovaries. The distinction between metastatic and independent tumors is significant since it affects staging and prognosis [1].
The distinction between metastatic and double primary tumors is difficult. Immunohistochemical and DNA flow cytometric studies may be of value for the distinction between metastatic and double primary tumor tumors [4]. While there is recent evidence that clonality assays may help distinguish between the two clinicopathologic situations [5]. The combination of different methods and the interpretation of molecular data in concordance with the clinicopathologic findings will probably provide useful information for these cases. While the etiology of these tumors remains unclear. Lynch et al. [6] reported that the occurrence of multiple primary malignant neoplasms characterizes virtually all varieties of hereditary cancer. It has also been postulated that embryologically similar tissues of the female genital tract when simultaneously subjected to carcinogens may develop synchronous neoplasms. Others suggest that these neoplasms originate in metaplasia occurring in histologically similar epithelium of the genital tract and peritoneum [7]. As the cancer associated with uterine endometrial cancer, ovarian cancer has been reported most prevalently. Cases such as our case that uterine endometrial cancer is associated with bladder cancer are very rare. Even more the presence of poor differentiation in the bladder carcinoma and the deep myometrial invasion found in our case is unusual.
Therefore, until now, the standardized guideline for its diagnosis or treatments has not been established yet, and it is treated by the methods suitable to each cancer. Since the incidence is very low, treatment responses or prognostic factors have not been reported yet. However, according to the reports, the prognosis in most cases of double primary malignant tumor is surprisingly good. It is reported that the prognosis of the "highly malignant" tumor does not worsen as a result of a second malignant cancer [8] and furthermore the prognosis of double primary carcinoma of uterine corpus and ovary is rather good [9,10]. The precise mechanism of good prognosis of double primary cancer has not been revealed yet. It just is speculated that in double primary cancer, only one cancer is diagnosed, and during the test procedure, another cancer may be detected and thus it is detected rather early, hence, the prognosis is good.
Engine [11] suggested prognosis of multiple primary cancer more comprehensively. According to his report, gender, age, and the interval of two primary tumors were prognostic factors. In our case, the prognosis is speculated to be poorer. Because the first tumor was detected after the age of 50 years, and the interval of the two primary cancers was less than 2 years. Furthermore the differentiation of bladder cancer was poor and it progressed rapidly.
Patients with endometrial cancer should be carefully and regularly followed up by monitoring et every anatomic site, especially the breast, stomach, and colon, in order that the development of a second primary carcinoma can be detected as early as possible, and not be overlooked in examinations. Additional risk factors for endometrial carcinoma with multiple malignant neoplasms include: menopause occurring after age fifty-one; obese women with body mass index higher than 32; reproductive period longer than 37 years [12]. Our case was a patient with early stage uterine endometrial cancer of which possibility of the metastasis to the bladder is very low. Endometrial cancer was treated and during follow-ups by general tests, bladder cancer was diagnosed. During postsurgical radiation therapy, our patient presented with intermittent hematuria, nevertheless, it was misdiagnosed as side effects of radiation therapy, and thus the diagnosis was delayed. In addition, our patient received total hip replacement surgery and her condition was inappropriate to diagnose masses in the bladder by imaging study. After uterine endometrial cancer treatments, the recurrence of uterine endometrial cancer was followed up by PET-CT, hence, bladder cancer was overlooked.
The patient was old and did not want to receive the total cystectomy, and thus only conservative management was performed with TUR-BT. Contrary to our expectations, her condition is tolerable except intermittent hematuria.
Figures and Tables
Fig. 1
(A) Pelvic magnetic resonance imaging. About 2.8×2.6×2.4 cm sized T2 intermediate signal intensity mass (arrow) within the endometrial cavity and thinning of myometrium, especially right side body and fundus. (B) Pelvic computed tomography. Irregular lobulated tumor (arrow) around right posterior inferior wall of the urinary bladder with suspicious adjacent perivascular retroperitoneal space infiltration along the right internal iliac vessels.
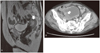
Fig. 2
In the uterus, typical endometrioid adenocarcinoma invading myometrium is noted (A). The lesion in the urinary bladder is morphologically different from that of the uterus, and composed of highly pleomorphic malignant cells without forming glandular structure (B). Immunohistologically, these tumor cells show positive reaction to cytokeratin (CK)-7 (C) and vimentin (D), but negative reaction to CK20 (E). So, for the lesion in the urinary bladder, poorly differentiated carcinoma or carcinosarcoma is suspected. Magnification, ×200.
References
1. Sheu BC, Lin HH, Chen CK, Chao KH, Shun CT, Huang SC. Synchronous primary carcinomas of the endometrium and ovary. Int J Gynaecol Obstet. 1995. 51:141–146.
2. Eisner RF, Nieberg RK, Berek JS. Synchronous primary neoplasms of the female reproductive tract. Gynecol Oncol. 1989. 33:335–339.
3. Castro IM, Connell PP, Waggoner S, Rotmensch J, Mundt AJ. Synchronous ovarian and endometrial malignancies. Am J Clin Oncol. 2000. 23:521–525.
4. Prat J, Matias-Guiu X, Barreto J. Simultaneous carcinoma involving the endometrium and the ovary. A clinicopathologic, immunohistochemical, and DNA flow cytometric study of 18 cases. Cancer. 1991. 68:2455–2459.
5. Prat J. Clonality analysis in synchronous tumors of the female genital tract. Hum Pathol. 2002. 33:383–385.
6. Lynch HT, Harris RE, Lynch PM, Guirgis HA, Lynch JF, Bardawil WA. Role of heredity in multiple primary cancer. Cancer. 1977. 40:1849–1854.
7. Ayhan A, Yalcin OT, Tuncer ZS, Gürgan T, Küçükali T. Synchronous primary malignancies of the female genital tract. Eur J Obstet Gynecol Reprod Biol. 1992. 45:63–66.
8. Schröcksnadel H, Fuith LC, Hetzel H. Multiple cancers in gynecologic oncology. Geburtshilfe Frauenheilkd. 1988. 48:710–714.
9. Chen F, Shen K, Lang JH, Huang HF, Wu M. Clinical features and prognostic of double primary carcinoma of uterine corpus and the ovary. Zhonghua Yi Xue Za Zhi. 2005. 85:1257–1260.
10. Papathanasiou K, Tolikas A, Dovas D, Kostopoulou E, Fragkedakis N, Tzafettas J. Simultaneously detected primary malignant tumors of ovary and endometrium with unusual histology. Int J Gynecol Cancer. 2005. 15:1191–1194.
11. Engin K. Cancers in multiple primary sites. Int Surg. 1994. 79:33–37.
12. Studziński Z, Branicka D. The coexistence of endometrial cancer with second primary malignant neoplasms. Ginekol Pol. 1999. 70:186–192.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download